当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
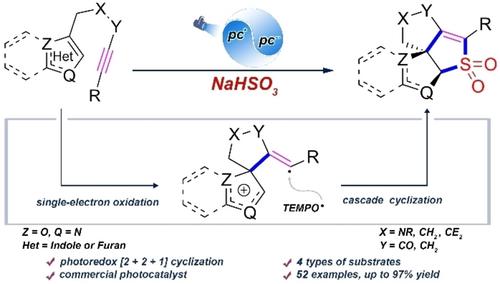
Visible-Light-Induced [2+2+1] Dearomative Cascade Cyclization of Indole/Furan Alkynes to Synthesize Sulfonyl Polycycles
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-12 , DOI: 10.1002/adsc.202200331 Jiajun Luo 1 , Guohui Zeng 1 , Xiaohui Cao 2 , Biaolin Yin 3
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-12 , DOI: 10.1002/adsc.202200331 Jiajun Luo 1 , Guohui Zeng 1 , Xiaohui Cao 2 , Biaolin Yin 3
Affiliation
|
Herein, we report a visible-light-induced [2+2+1] dearomative cascade cyclization of indole/furan alkynes with NaHSO3, providing an array of diverse highly strained sulfonyl polycycles. Compared to our previous work, this method does not require the use of additional sacrificial oxidants and have wider reaction scope. Preliminary mechanistic studies suggest that the indole moiety initiated the reaction, which proceeded via single-electron oxidation pathway. An alkenyl radical formed by intramolecular addition capture SO2, and the resulting specie is cyclized to furnish the final product. Also, DFT calculations disclosed that the groups on the indole ring or at the side chain probably affect the single electron distribution at the reaction site of IM-RC-I, and the distribution may be a decisive factor of spirocyclization.
中文翻译:

可见光诱导[2+2+1]脱芳构级联环化吲哚/呋喃炔烃合成磺酰基多环化合物
在此,我们报道了可见光诱导的吲哚/呋喃炔烃与 NaHSO 3的 [2+2+1] 脱芳族级联环化,提供了一系列不同的高应变磺酰基多环化合物。与我们之前的工作相比,该方法不需要使用额外的牺牲氧化剂,反应范围更广。初步机理研究表明,吲哚部分引发了反应,该反应通过单电子氧化途径进行。分子内加成捕获SO 2形成的烯基自由基, 并将所得物质环化以提供最终产品。此外,DFT 计算表明,吲哚环或侧链上的基团可能影响 IM-RC-I 反应位点的单电子分布,该分布可能是螺环化的决定性因素。
更新日期:2022-05-12
中文翻译:

可见光诱导[2+2+1]脱芳构级联环化吲哚/呋喃炔烃合成磺酰基多环化合物
在此,我们报道了可见光诱导的吲哚/呋喃炔烃与 NaHSO 3的 [2+2+1] 脱芳族级联环化,提供了一系列不同的高应变磺酰基多环化合物。与我们之前的工作相比,该方法不需要使用额外的牺牲氧化剂,反应范围更广。初步机理研究表明,吲哚部分引发了反应,该反应通过单电子氧化途径进行。分子内加成捕获SO 2形成的烯基自由基, 并将所得物质环化以提供最终产品。此外,DFT 计算表明,吲哚环或侧链上的基团可能影响 IM-RC-I 反应位点的单电子分布,该分布可能是螺环化的决定性因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号