Chem Catalysis Pub Date : 2022-05-11 , DOI: 10.1016/j.checat.2022.04.012 Hassina Tabassum 1, 2 , Xiaoxuan Yang 1 , Ruqiang Zou 2 , Gang Wu 1
|
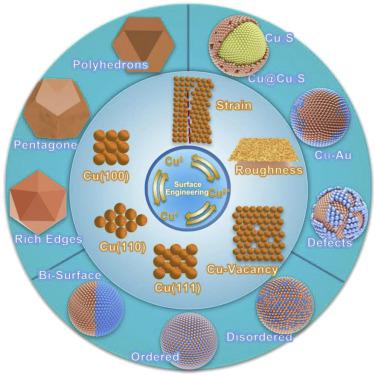
Copper (Cu) is the most efficient metal that can electrochemically convert CO2 to various chemical feedstocks at reasonable efficiency. The activity and selectivity toward the CO2 reduction reaction (CO2RR) largely depend on the surface sensitivity and electrokinetics of Cu catalysts. Surface engineering is achievable through tuning the structure and through crystal orientation. The Cu-surface modulation and tunings, e.g., controlled morphology, oxygen vacancies, and alloys on supports or substrates, propose different reaction tracks and intermediates, whereas common routes are ∗CO dimerization, C–C, and C1–C2 coupling for the formation of C2 and C3 products. In this review, recent progress on the surface engineering of Cu-based catalysts is primarily recaptured and explained. The fragmentation, coalescence, and aggregation of Cu nanoparticles cause stability issues of Cu catalysts during the CO2RR, which has also been discussed. Finally, we summarize critical strategies and approaches to surface engineering of Cu-based catalysts for the efficient CO2RR.
中文翻译:

用于将 CO2 电化学还原为增值多碳产品的 Cu 催化剂的表面工程
铜 (Cu) 是最有效的金属,能够以合理的效率将 CO 2电化学转化为各种化学原料。CO 2还原反应(CO 2 RR)的活性和选择性很大程度上取决于Cu催化剂的表面敏感性和电动力学。表面工程可以通过调整结构和晶体取向来实现。Cu 表面调制和调整,例如受控形态、氧空位和载体或基板上的合金,提出了不同的反应轨迹和中间体,而常见的途径是*CO 二聚化、C-C 和 C 1 -C 2耦合C 2和 C 3的形成产品。在这篇综述中,主要回顾和解释了铜基催化剂表面工程的最新进展。Cu纳米颗粒的碎裂、聚结和聚集会导致Cu催化剂在CO 2 RR过程中的稳定性问题,对此也进行了讨论。最后,我们总结了用于高效 CO 2 RR的铜基催化剂表面工程的关键策略和方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号