当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ether-based electrolytes for sodium ion batteries
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2022-05-11 , DOI: 10.1039/d1cs00948f Ying Li 1 , Feng Wu 1, 2 , Yu Li 1 , Mingquan Liu 1, 2 , Xin Feng 1 , Ying Bai 1 , Chuan Wu 1, 2
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2022-05-11 , DOI: 10.1039/d1cs00948f Ying Li 1 , Feng Wu 1, 2 , Yu Li 1 , Mingquan Liu 1, 2 , Xin Feng 1 , Ying Bai 1 , Chuan Wu 1, 2
Affiliation
|
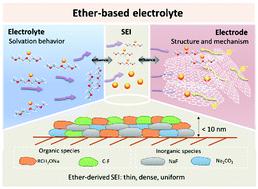
Sodium-ion batteries (SIBs) are considered to be strong candidates for large-scale energy storage with the benefits of cost-effectiveness and sodium abundance. Reliable electrolytes, as ionic conductors that regulate the electrochemical reaction behavior and the nature of the interface and electrode, are indispensable in the development of advanced SIBs with high Coulombic efficiency, stable cycling performance and high rate capability. Conventional carbonate-based electrolytes encounter numerous obstacles for their wide application in SIBs due to the formation of a dissolvable, continuous-thickening solid electrolyte interface (SEI) layer and inferior stability with electrodes. Comparatively, ether-based electrolytes (EBEs) are emerging in the secondary battery field with fascinating properties to improve the performance of batteries, especially SIBs. Their stable solvation structure enables highly reversible solvent-co-intercalation reactions and the formation of a thin and stable SEI. However, although EBEs can provide more stable cycling and rapid sodiation kinetics in electrodes, benefitting from their favorable electrolyte/electrode interactions such as chemical compatibility and good wettability, their special chemistry is still being investigated and puzzling. In this review, we provide a thorough and comprehensive overview on the developmental history, fundamental characteristics, superiorities and mechanisms of EBEs, together with their advances in other battery systems. Notably, the relation among electrolyte science, interfacial chemistry and electrochemical performance is highlighted, which is of great significance for the in-depth understanding of battery chemistry. Finally, future perspectives and potential directions are proposed to navigate the design and optimization of electrolytes and electrolyte/electrode interfaces for advanced batteries.
中文翻译:

钠离子电池用醚基电解质
钠离子电池(SIB)被认为是大规模储能的有力候选者,具有成本效益和钠丰富的优势。可靠的电解质作为调节电化学反应行为以及界面和电极性质的离子导体,对于开发具有高库仑效率、稳定循环性能和高倍率性能的先进 SIB 是必不可少的。由于形成可溶解的、连续增厚的固体电解质界面(SEI)层和电极稳定性差,传统的碳酸盐基电解质在 SIB 中的广泛应用遇到了许多障碍。相比之下,醚基电解质(EBEs)在二次电池领域正在兴起,具有提高电池性能的迷人特性,尤其是 SIB。它们稳定的溶剂化结构能够实现高度可逆的溶剂共嵌入反应并形成薄而稳定的 SEI。然而,尽管 EBE 可以在电极中提供更稳定的循环和快速的钠化动力学,受益于它们有利的电解质/电极相互作用,例如化学相容性和良好的润湿性,但它们的特殊化学性质仍在研究中并令人费解。在这篇综述中,我们对 EBE 的发展历史、基本特征、优势和机制以及它们在其他电池系统中的进展进行了全面而全面的概述。值得注意的是,强调了电解质科学、界面化学和电化学性能之间的关系,这对于深入了解电池化学具有重要意义。最后,提出了未来的观点和潜在方向,以指导先进电池的电解质和电解质/电极界面的设计和优化。
更新日期:2022-05-11
中文翻译:

钠离子电池用醚基电解质
钠离子电池(SIB)被认为是大规模储能的有力候选者,具有成本效益和钠丰富的优势。可靠的电解质作为调节电化学反应行为以及界面和电极性质的离子导体,对于开发具有高库仑效率、稳定循环性能和高倍率性能的先进 SIB 是必不可少的。由于形成可溶解的、连续增厚的固体电解质界面(SEI)层和电极稳定性差,传统的碳酸盐基电解质在 SIB 中的广泛应用遇到了许多障碍。相比之下,醚基电解质(EBEs)在二次电池领域正在兴起,具有提高电池性能的迷人特性,尤其是 SIB。它们稳定的溶剂化结构能够实现高度可逆的溶剂共嵌入反应并形成薄而稳定的 SEI。然而,尽管 EBE 可以在电极中提供更稳定的循环和快速的钠化动力学,受益于它们有利的电解质/电极相互作用,例如化学相容性和良好的润湿性,但它们的特殊化学性质仍在研究中并令人费解。在这篇综述中,我们对 EBE 的发展历史、基本特征、优势和机制以及它们在其他电池系统中的进展进行了全面而全面的概述。值得注意的是,强调了电解质科学、界面化学和电化学性能之间的关系,这对于深入了解电池化学具有重要意义。最后,提出了未来的观点和潜在方向,以指导先进电池的电解质和电解质/电极界面的设计和优化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号