当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Organocatalyzed Friedel–Crafts Reaction of Trihaloacetaldehydes and Phenols
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-10 , DOI: 10.1002/adsc.202200180 David Svestka 1 , Jan Otevrel 1 , Pavel Bobal 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-05-10 , DOI: 10.1002/adsc.202200180 David Svestka 1 , Jan Otevrel 1 , Pavel Bobal 1
Affiliation
|
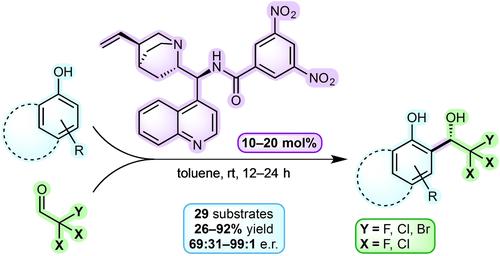
Herein we report the asymmetric organocatalyzed method for the Friedel–Crafts reaction between activated phenols and trihaloacetaldehydes. A three-phase screening including 41 compounds was employed to identify a catalyst structure based on 3,5-dinitrobenzamide of 9-amino-epi-cinchonidine as the lead catalytic molecule. Under the optimized reaction conditions, the above catalyst offered trihalohydroxyalkylated adducts in yields ranging from 26 to 92% and enantiomeric ratios within 69:31–99:1. The reaction scope was determined on 29 entries and several follow-up transformations of the enantioenriched products were accomplished.
中文翻译:

三卤代乙醛和酚的不对称有机催化傅克反应
在这里,我们报告了活化酚和三卤代乙醛之间的傅克反应的不对称有机催化方法。采用包括 41 种化合物的三相筛选来确定基于 9-氨基-表-辛可尼丁的 3,5-二硝基苯甲酰胺作为先导催化分子的催化剂结构。在优化的反应条件下,上述催化剂提供的三卤代羟基烷基化加合物的收率在 26% 到 92% 之间,对映体比在 69:31-99:1 之间。确定了29个条目的反应范围,并完成了对映体富集产物的多次后续转化。
更新日期:2022-05-10
中文翻译:

三卤代乙醛和酚的不对称有机催化傅克反应
在这里,我们报告了活化酚和三卤代乙醛之间的傅克反应的不对称有机催化方法。采用包括 41 种化合物的三相筛选来确定基于 9-氨基-表-辛可尼丁的 3,5-二硝基苯甲酰胺作为先导催化分子的催化剂结构。在优化的反应条件下,上述催化剂提供的三卤代羟基烷基化加合物的收率在 26% 到 92% 之间,对映体比在 69:31-99:1 之间。确定了29个条目的反应范围,并完成了对映体富集产物的多次后续转化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号