当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quinolinate Synthase: An Example of the Roles of the Second and Outer Coordination Spheres in Enzyme Catalysis
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.chemrev.1c00869 Juan C Fontecilla-Camps 1 , Anne Volbeda 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.chemrev.1c00869 Juan C Fontecilla-Camps 1 , Anne Volbeda 1
Affiliation
|
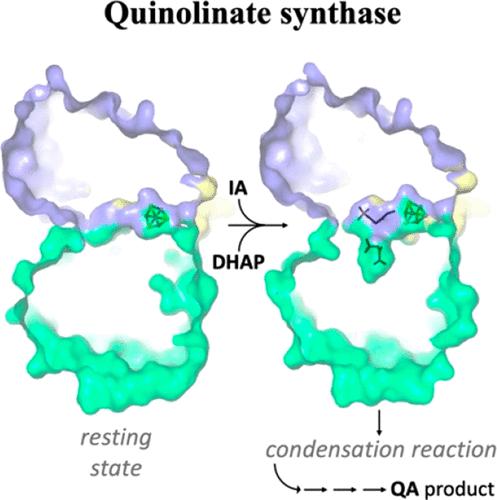
The activation energy barrier of biochemical reactions is normally lowered by an enzyme catalyst, which directly helps the weakening of the bond(s) to be broken. In many metalloenzymes, this is a first coordination sphere effect. Besides having a direct catalytic action, enzymes can fix their reactive groups and substrates so that they are optimally positioned and also modify the water activity in the system. They can either activate substrates prior to their reaction or bind preactivated substrates, thereby drastically reducing local entropic effects. The latter type is well represented by some bisubstrate reactions, where they have been defined as “entropic traps”. These can be described as “second coordination sphere” processes, but enzymes can also control the reactivity beyond this point through local conformational changes belonging to an “outer coordinate sphere” that can be modulated by substrate binding. We have chosen the [4Fe-4S] cluster-dependent enzyme quinolinate synthase to illustrate each one of these processes. In addition, this very old metalloenzyme shows low in vitro substrate binding specificity, atypical reactivity that produces dead-end products, and a unique modulation of its active site volume.
中文翻译:

喹啉酸合酶:第二和外配位球在酶催化中的作用的一个例子
生化反应的活化能垒通常由酶催化剂降低,这直接有助于弱化键的断裂。在许多金属酶中,这是第一个配位球效应。除了具有直接的催化作用外,酶还可以固定它们的反应基团和底物,使它们处于最佳位置,也可以改变系统中的水活性。它们既可以在反应之前激活底物,也可以结合预激活的底物,从而大大降低局部熵效应。后一种类型很好地代表了一些双底物反应,它们被定义为“熵陷阱”。这些可以被描述为“第二协调领域”过程,但酶还可以通过属于“外部坐标球”的局部构象变化来控制超出这一点的反应性,该“外部坐标球”可以通过底物结合进行调节。我们选择了 [4Fe-4S] 簇依赖性酶喹啉酸合酶来说明这些过程中的每一个。此外,这种非常古老的金属酶显示出低体外底物结合特异性、产生死端产物的非典型反应性以及对其活性位点体积的独特调节。
更新日期:2022-05-10
中文翻译:

喹啉酸合酶:第二和外配位球在酶催化中的作用的一个例子
生化反应的活化能垒通常由酶催化剂降低,这直接有助于弱化键的断裂。在许多金属酶中,这是第一个配位球效应。除了具有直接的催化作用外,酶还可以固定它们的反应基团和底物,使它们处于最佳位置,也可以改变系统中的水活性。它们既可以在反应之前激活底物,也可以结合预激活的底物,从而大大降低局部熵效应。后一种类型很好地代表了一些双底物反应,它们被定义为“熵陷阱”。这些可以被描述为“第二协调领域”过程,但酶还可以通过属于“外部坐标球”的局部构象变化来控制超出这一点的反应性,该“外部坐标球”可以通过底物结合进行调节。我们选择了 [4Fe-4S] 簇依赖性酶喹啉酸合酶来说明这些过程中的每一个。此外,这种非常古老的金属酶显示出低体外底物结合特异性、产生死端产物的非典型反应性以及对其活性位点体积的独特调节。


























 京公网安备 11010802027423号
京公网安备 11010802027423号