Chem ( IF 23.5 ) Pub Date : 2022-05-06 , DOI: 10.1016/j.chempr.2022.04.011 Guang-Jian Mei 1 , Wai Lean Koay 2 , Chun-Yan Guan 1 , Yixin Lu 2
|
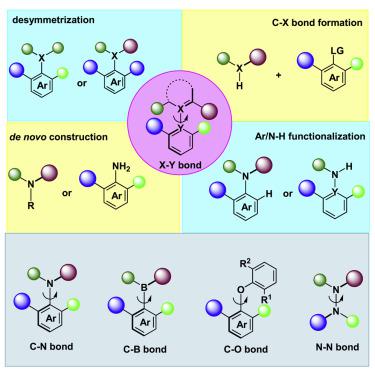
Atropisomers beyond the C–C axis (denoted as X–Y herein) are important addition to the repertoire of axially chiral compounds, which have received much attention in recent years. Compared with conventional C–C axial chirality around biaryl and olefin axes, atropisomerism portrayed by C–N, C–O, C–B, or N–N bond was deemed to be challenging due to the relatively low rotational barriers. However, the intrinsic shorter bond length and electron-repelling effect lead to a congested hetero X–Y axis, resulting in stable axially chiral frameworks. The past two decades, especially the past few years have witnessed a rapid progress of this emerging area. A range of catalytic atroposelective approaches have been reported for the efficient synthesis of these challenging skeletons. The X–Y axially chiral compounds are valuable molecules, and they may be used as new ligands or catalysts in asymmetric catalysis or evaluated for their potential biological activities. We believe that the chemistry of atropisomers beyond C–C axial chirality will be forthcoming and blooming in the near future, taking up an important position in organic chemistry and beyond.
中文翻译:

超越 C-C 轴向手性的阻转异构体:催化不对称合成的进展
C-C 轴以外的阻转异构体(此处表示为 X-Y)是轴向手性化合物库的重要补充,近年来备受关注。与传统的围绕联芳基和烯烃轴的 C-C 轴向手性相比,由于旋转势垒相对较低,由 C-N、C-O、C-B 或 N-N 键描绘的阻转异构被认为具有挑战性。然而,固有的较短键长和电子排斥效应导致拥挤的异质 X-Y 轴,从而产生稳定的轴向手性框架。过去的二十年,尤其是过去的几年,见证了这一新兴领域的快速发展。已经报道了用于有效合成这些具有挑战性的骨架的一系列催化抗转选择性方法。X-Y 轴向手性化合物是有价值的分子,它们可用作不对称催化中的新配体或催化剂,或评估其潜在的生物活性。我们相信,超越C-C轴向手性的阻转异构体化学将在不久的将来出现并大放异彩,在有机化学及其他领域占据重要地位。


























 京公网安备 11010802027423号
京公网安备 11010802027423号