当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optimization of Metal Affinity Ketoreductase Immobilization for Application in Batch and Flow Processes
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-05-04 , DOI: 10.1021/acs.oprd.1c00483 Alessandra Basso 1 , Maria S. Brown 2 , Alvaro Cruz-Izquierdo 1 , Carlos A. Martinez 2 , Simona Serban 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-05-04 , DOI: 10.1021/acs.oprd.1c00483 Alessandra Basso 1 , Maria S. Brown 2 , Alvaro Cruz-Izquierdo 1 , Carlos A. Martinez 2 , Simona Serban 1
Affiliation
|
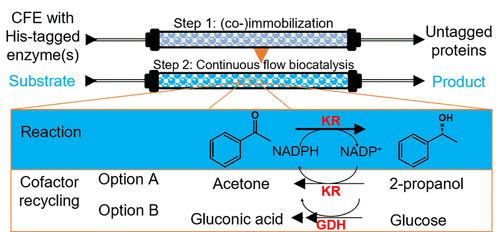
Protein isolation from fermentation broth or protein mixtures by using cost-effective, scalable, regenerable resins offers a significant increase in value and productivity; hence, many recombinant proteins contain a fused histidine tag to facilitate immobilized metal affinity chromatography purification. A newly developed methacrylic iminodiacetic acid-functionalized resin, capable of attaining metal ion loadings of 0.25 mmol metal/g and high affinity toward His-tagged protein, was studied in the immobilization of a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent His-tagged ketoreductase and in the coimmobilization of the ketoreductase and glucose dehydrogenase. The affinity immobilization was compared to various other resin-enzyme interactions, that is, covalent, ionic, and hydrophobic, showing that the His-tag immobilization provides up to 4 times increase in specific activity due to the high immobilization selectivity and suitable enzyme orientation. Biocatalyst recycling in batch showed excellent stability over 10 cycles in aqueous and 5 cycles in organic media. NADPH cofactor recycling was studied in batch and continuous flow using cosubstrate 2-propanol as well as separately immobilized and coimmobilized ketoreductase and glucose dehydrogenase, in the presence of glucose. The best biotransformation with cofactor regeneration was in the presence of the coimmobilized ketoreductase and glucose dehydrogenase, while the lowest conversion was obtained by using the two enzymes separately immobilized. These studies showed that, by using cofactor regeneration systems, an NADPH concentration 1000 times lower than the substrate concentration can be used while maintaining the enzyme productivity.
中文翻译:

金属亲和酮还原酶固定化的优化在批处理和流动过程中的应用
通过使用具有成本效益、可扩展、可再生的树脂从发酵液或蛋白质混合物中分离蛋白质可显着提高价值和生产力;因此,许多重组蛋白含有融合组氨酸标签,以促进固定化金属亲和层析纯化。研究了一种新开发的甲基丙烯酸亚氨基二乙酸功能化树脂,能够实现 0.25 mmol 金属/g 的金属离子负载量和对 His 标签蛋白的高亲和力,用于固定还原型烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 依赖性 His-标记的酮还原酶和在酮还原酶和葡萄糖脱氢酶的共固定化中。将亲和固定与各种其他树脂-酶相互作用(即共价、离子和疏水)进行了比较,表明由于高固定选择性和合适的酶方向,His 标签固定可提供高达 4 倍的比活性增加。批量生物催化剂回收在水介质中 10 次循环和在有机介质中 5 次循环后表现出优异的稳定性。在葡萄糖存在下,使用共底物 2-丙醇以及分别固定和共固定的酮还原酶和葡萄糖脱氢酶分批和连续流动研究 NADPH 辅因子回收。辅因子再生的最佳生物转化是在共固定化酮还原酶和葡萄糖脱氢酶存在的情况下,而使用分别固定化的两种酶获得的转化率最低。这些研究表明,通过使用辅因子再生系统,
更新日期:2022-05-04
中文翻译:

金属亲和酮还原酶固定化的优化在批处理和流动过程中的应用
通过使用具有成本效益、可扩展、可再生的树脂从发酵液或蛋白质混合物中分离蛋白质可显着提高价值和生产力;因此,许多重组蛋白含有融合组氨酸标签,以促进固定化金属亲和层析纯化。研究了一种新开发的甲基丙烯酸亚氨基二乙酸功能化树脂,能够实现 0.25 mmol 金属/g 的金属离子负载量和对 His 标签蛋白的高亲和力,用于固定还原型烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 依赖性 His-标记的酮还原酶和在酮还原酶和葡萄糖脱氢酶的共固定化中。将亲和固定与各种其他树脂-酶相互作用(即共价、离子和疏水)进行了比较,表明由于高固定选择性和合适的酶方向,His 标签固定可提供高达 4 倍的比活性增加。批量生物催化剂回收在水介质中 10 次循环和在有机介质中 5 次循环后表现出优异的稳定性。在葡萄糖存在下,使用共底物 2-丙醇以及分别固定和共固定的酮还原酶和葡萄糖脱氢酶分批和连续流动研究 NADPH 辅因子回收。辅因子再生的最佳生物转化是在共固定化酮还原酶和葡萄糖脱氢酶存在的情况下,而使用分别固定化的两种酶获得的转化率最低。这些研究表明,通过使用辅因子再生系统,



























 京公网安备 11010802027423号
京公网安备 11010802027423号