Cell ( IF 64.5 ) Pub Date : 2022-04-27 , DOI: 10.1016/j.cell.2022.03.042 Wesley B Asher 1 , Daniel S Terry 2 , G Glenn A Gregorio 3 , Alem W Kahsai 4 , Alessandro Borgia 2 , Bing Xie 5 , Arnab Modak 2 , Ying Zhu 1 , Wonjo Jang 6 , Alekhya Govindaraju 7 , Li-Yin Huang 4 , Asuka Inoue 8 , Nevin A Lambert 6 , Vsevolod V Gurevich 9 , Lei Shi 5 , Robert J Lefkowitz 10 , Scott C Blanchard 11 , Jonathan A Javitch 12
|
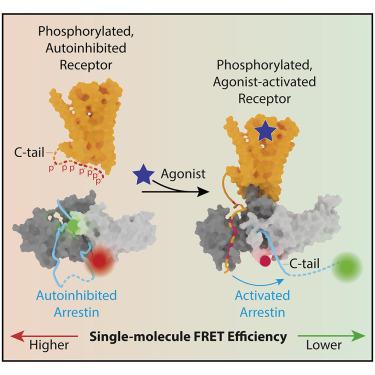
β-arrestins bind G protein-coupled receptors to terminate G protein signaling and to facilitate other downstream signaling pathways. Using single-molecule fluorescence resonance energy transfer imaging, we show that β-arrestin is strongly autoinhibited in its basal state. Its engagement with a phosphopeptide mimicking phosphorylated receptor tail efficiently releases the β-arrestin tail from its N domain to assume distinct conformations. Unexpectedly, we find that β-arrestin binding to phosphorylated receptor, with a phosphorylation barcode identical to the isolated phosphopeptide, is highly inefficient and that agonist-promoted receptor activation is required for β-arrestin activation, consistent with the release of a sequestered receptor C tail. These findings, together with focused cellular investigations, reveal that agonism and receptor C-tail release are specific determinants of the rate and efficiency of β-arrestin activation by phosphorylated receptor. We infer that receptor phosphorylation patterns, in combination with receptor agonism, synergistically establish the strength and specificity with which diverse, downstream β-arrestin-mediated events are directed.
中文翻译:

GPCR 介导的 β-arrestin 激活以单分子精度解卷积
β-抑制蛋白结合 G 蛋白偶联受体以终止 G 蛋白信号传导并促进其他下游信号通路。使用单分子荧光共振能量转移成像,我们表明 β-arrestin 在其基础状态下具有强烈的自抑制作用。它与模拟磷酸化受体尾部的磷酸肽的结合有效地从其 N 结构域释放 β-arrestin 尾部以呈现不同的构象。出乎意料的是,我们发现 β-arrestin 与磷酸化受体结合,其磷酸化条形码与分离的磷酸肽相同,效率非常低,并且 β-arrestin 激活需要激动剂促进的受体激活,这与隔离受体 C 的释放一致尾巴。这些发现连同重点细胞研究,揭示激动作用和受体 C 尾释放是磷酸化受体激活 β-抑制蛋白的速率和效率的特定决定因素。我们推断,受体磷酸化模式与受体激动作用相结合,协同确定了多种下游 β-arrestin 介导事件的强度和特异性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号