当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A theoretical investigation into gallic acid pyrolysis
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-04-22 , DOI: 10.1002/jcc.26865 Jakob Kraus 1 , Jens Kortus 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-04-22 , DOI: 10.1002/jcc.26865 Jakob Kraus 1 , Jens Kortus 1
Affiliation
|
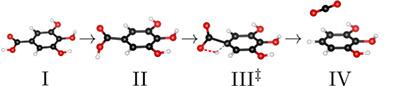
Thermodynamic and kinetic information on the first two steps of gallic acid pyrolysis, a decarboxylation followed by a dehydrogenation, is obtained based on density functional theory and quantum chemistry. For the kinetics, transition states are identified with the help of the climbing image nudged elastic band method. Both reactions exhibit two transition states. One of them is related to the rotation of OH groups, and the other one is related to the breaking and forming of bonds. The gallic acid pyrolysis as a whole is judged to be endothermal, and it changes from endergonic to exergonic between 500 and 750 K. The second reaction, the dehydrogenation of pyrogallol, is identified as the rate-determining step of gallic acid pyrolysis, with reaction rate constants below 1 s−1 for temperatures below 1250 K.
中文翻译:

没食子酸热解的理论研究
基于密度泛函理论和量子化学,获得了没食子酸热解前两个步骤(脱羧后脱氢)的热力学和动力学信息。对于动力学,借助攀爬图像轻推弹性带方法识别过渡态。两种反应都表现出两种过渡态。其中一个与 OH 基团的旋转有关,另一个与键的断裂和形成有关。没食子酸热解作为一个整体被判断为吸热的,它在 500 和 750 K 之间从吸能转变为放能。对于低于 1250 K 的温度,速率常数低于 1 s -1 。
更新日期:2022-04-22
中文翻译:

没食子酸热解的理论研究
基于密度泛函理论和量子化学,获得了没食子酸热解前两个步骤(脱羧后脱氢)的热力学和动力学信息。对于动力学,借助攀爬图像轻推弹性带方法识别过渡态。两种反应都表现出两种过渡态。其中一个与 OH 基团的旋转有关,另一个与键的断裂和形成有关。没食子酸热解作为一个整体被判断为吸热的,它在 500 和 750 K 之间从吸能转变为放能。对于低于 1250 K 的温度,速率常数低于 1 s -1 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号