Structure ( IF 5.7 ) Pub Date : 2022-04-22 , DOI: 10.1016/j.str.2022.03.017 Manmohan Sharma 1 , Nachiappan Mutharasappan 2 , Yogavel Manickam 2 , Karl Harlos 3 , Bruno Melillo 4 , Eamon Comer 5 , Heena Tabassum 6 , Suhel Parvez 7 , Stuart L Schreiber 8 , Amit Sharma 9
|
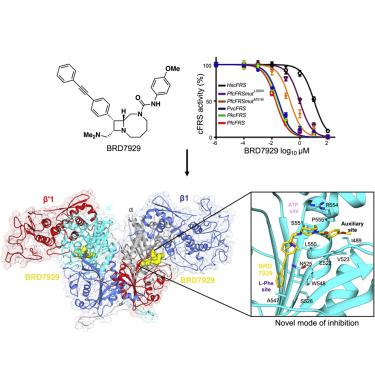
Bicyclic azetidine compounds possess antimalarial activity via targeting of the cytoplasmic Plasmodium falciparum (Pf) protein translation enzyme phenylalanine-tRNA synthetase (cFRS). These drugs kill parasites both in vitro and in vivo, including the blood, liver, and transmission developmental stages. Here we present the co-crystal structure of PfcFRS with a potent inhibitor, the bicyclic azetidine BRD7929. Our studies reveal high-affinity binding of BRD7929 with PfcFRS along with exquisite specificity compared with the human enzyme, leading in turn to potent and selective inhibition of the parasite enzyme. Our co-crystal structure shows that BRD7929 binds in the active site in the α subunit of PfcFRS, where it occupies the amino acid site, an auxiliary site, and partially the ATP site. This structural snapshot of inhibitor-bound PfcFRS thus provides a platform for the structure-guided optimization of novel antimalarial compounds.
中文翻译:

抑制恶性疟原虫苯丙氨酸 tRNA 合成酶为抗疟药物开发提供了机会
双环氮杂环丁烷化合物通过靶向细胞质恶性疟原虫( Pf ) 蛋白翻译酶苯丙氨酸-tRNA 合成酶 (cFRS) 具有抗疟活性。这些药物在体外和体内杀死寄生虫,包括血液、肝脏和传播发育阶段。在这里,我们展示了Pf cFRS 与强效抑制剂双环氮杂环丁烷 BRD7929的共晶结构。我们的研究揭示了 BRD7929 与Pf的高亲和力结合与人类酶相比,cFRS 具有出色的特异性,进而导致对寄生虫酶的有效和选择性抑制。我们的共晶结构显示 BRD7929 结合在Pf cFRS 的 α 亚基的活性位点,它占据了氨基酸位点、辅助位点和部分 ATP 位点。因此,抑制剂结合的Pf cFRS 的这种结构快照为新型抗疟化合物的结构引导优化提供了一个平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号