Structure ( IF 5.7 ) Pub Date : 2022-04-22 , DOI: 10.1016/j.str.2022.03.018 Anthony N Hodder 1 , Janni Christensen 1 , Stephen Scally 1 , Tony Triglia 2 , Anna Ngo 2 , Richard W Birkinshaw 1 , Brodie Bailey 1 , Paola Favuzza 1 , Melanie H Dietrich 1 , Wai-Hong Tham 1 , Peter E Czabotar 1 , Kym Lowes 2 , Zhuyan Guo 3 , Nicholas Murgolo 3 , Manuel de Lera Ruiz 3 , John A McCauley 3 , Brad E Sleebs 1 , David Olsen 3 , Alan F Cowman 1
|
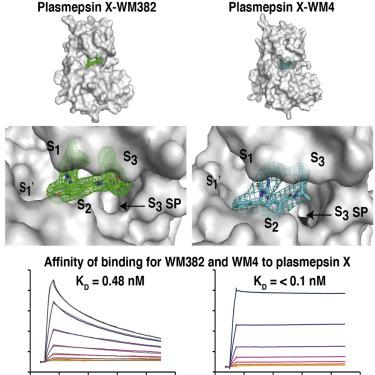
Plasmepsins IX (PMIX) and X (PMX) are essential aspartyl proteases for Plasmodium spp. egress, invasion, and development. WM4 and WM382 inhibit PMIX and PMX in Plasmodium falciparum and P. vivax. WM4 inhibits PMX, while WM382 is a dual inhibitor of PMIX and PMX. To understand their function, we identified protein substrates. Enzyme kinetic and structural analyses identified interactions responsible for drug specificity. PMIX and PMX have similar substrate specificity; however, there are distinct differences for peptide and protein substrates. Differences in WM4 and WM382 binding for PMIX and PMX map to variations in the S' region and engagement of the active site S3 pocket. Structures of PMX reveal interactions and mechanistic detail of drug binding important for development of clinical candidates against these targets.
中文翻译:

恶性疟原虫和间日疟原虫 IX 和 X 抑制的药物选择性基础
Plasmepsins IX (PMIX) 和 X (PMX) 是疟原虫属必需的天冬氨酰蛋白酶。出口、入侵和发展。WM4 和 WM382 抑制恶性疟原虫和间日疟原虫中的 PMIX 和 PMX. WM4 抑制 PMX,而 WM382 是 PMIX 和 PMX 的双重抑制剂。为了了解它们的功能,我们确定了蛋白质底物。酶动力学和结构分析确定了导致药物特异性的相互作用。PMIX 和 PMX 具有相似的底物特异性;然而,肽和蛋白质底物存在明显差异。WM4 和 WM382 与 PMIX 和 PMX 结合的差异映射到 S' 区域的变化和活性位点 S3 口袋的接合。PMX 的结构揭示了药物结合的相互作用和机制细节,这对于开发针对这些靶标的临床候选物很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号