Hydrometallurgy ( IF 4.7 ) Pub Date : 2022-04-11 , DOI: 10.1016/j.hydromet.2022.105871 Anna Wołowicz 1 , Zbigniew Hubicki 1
|
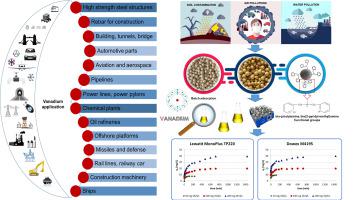
The present study deals with the potential application of the two adsorbents Dowex M4195 (M4195) and Lewatit MonoPlus TP220 (TP220) for the removal of vanadium(V) from the model and real aqueous solutions. The kinetic and equilibrium studies as well as the effects of pH, ion exchanger dose, contact time and initial concentration were studied using the batch method. Both the Langmuir and Freundlich adsorption isotherms and the pseudo-first order, pseudo-second order, intraparticle diffusion kinetic models were used to describe the adsorption process. The maximum adsorption capacity obtained from the Langmuir isotherm was found to be 248 mg/g and 209 mg/g for TP220 and M4195, respectively. The kinetics followed the pseudo second order reaction. Desorption, reusability of ion exchangers and vanadium(V) removal in the continuous system (column studies) were determined.
中文翻译:

离子交换树脂从模型和实际溶液中去除 V2O5 催化剂中的钒
本研究涉及两种吸附剂 Dowex M4195 (M4195) 和 Lewatit MonoPlus TP220 (TP220) 在从模型和实际水溶液中去除钒 (V) 的潜在应用。动力学和平衡研究以及 pH 值、离子交换剂剂量、接触时间和初始浓度的影响使用批处理法进行了研究。Langmuir 和 Freundlich 吸附等温线和准一级、准二级、颗粒内扩散动力学模型均用于描述吸附过程。TP220 和 M4195 从 Langmuir 等温线获得的最大吸附容量分别为 248 mg/g 和 209 mg/g。动力学遵循准二级反应。解吸,


























 京公网安备 11010802027423号
京公网安备 11010802027423号