Solid State Nuclear Magnetic Resonance ( IF 3.2 ) Pub Date : 2022-04-11 , DOI: 10.1016/j.ssnmr.2022.101794 Renny Mathew 1 , Ivan V Sergeyev 2 , Fabien Aussenac 3 , Lydia Gkoura 1 , Melanie Rosay 2 , Maria Baias 1
|
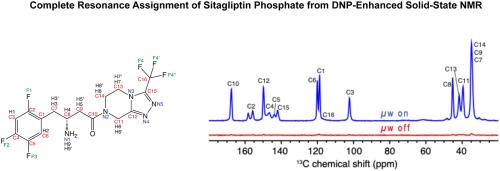
Solid-state dynamic nuclear polarization enhanced magic angle spinning (DNP-MAS) NMR measurements coupled with density functional theory (DFT) calculations enable the full resonance assignment of a complex pharmaceutical drug molecule without the need for isotopic enrichment. DNP dramatically enhances the NMR signals, thereby making possible previously intractable two-dimensional correlation NMR spectra at natural abundance. Using inputs from DFT calculations, herein we describe a significant improvement to the structure elucidation process for complex organic molecules. Further, we demonstrate that a series of two-dimensional correlation experiments, including 15N–13C TEDOR, 13C–13C INADEQUATE/SARCOSY, 19F–13C HETCOR, and 1H–13C HETCOR, can be obtained at natural isotopic abundance within reasonable experiment times, thus enabling a complete resonance assignment of sitagliptin, a pharmaceutical used for the treatment of type 2 diabetes.
中文翻译:

DNP 增强型固态 NMR 对药物在天然同位素丰度下的完全共振分配
固态动态核极化增强魔角旋转 (DNP-MAS) NMR 测量与密度泛函理论 (DFT) 计算相结合,无需同位素富集即可实现复杂药物分子的完全共振分配。DNP 显着增强了 NMR 信号,从而使以前难以处理的二维相关 NMR 光谱在自然丰度下成为可能。使用来自 DFT 计算的输入,我们在此描述了对复杂有机分子的结构解析过程的重大改进。此外,我们证明了一系列二维相关实验,包括15 N– 13 C TEDOR、13 C– 13 C INADEQUATE/SARCOSY、19 F–13 C HETCOR 和1 H– 13 C HETCOR 可以在合理的实验时间内以天然同位素丰度获得,从而使用于治疗 2 型糖尿病的药物西格列汀完全共振。


























 京公网安备 11010802027423号
京公网安备 11010802027423号