当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction Mechanisms, Kinetics, and Improved Catalysts for Ammonia Synthesis from Hierarchical High Throughput Catalyst Design
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-04-06 , DOI: 10.1021/acs.accounts.1c00789 Jon Fuller 1 , Qi An 1 , Alessandro Fortunelli 2, 3 , William A Goddard 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-04-06 , DOI: 10.1021/acs.accounts.1c00789 Jon Fuller 1 , Qi An 1 , Alessandro Fortunelli 2, 3 , William A Goddard 2
Affiliation
|
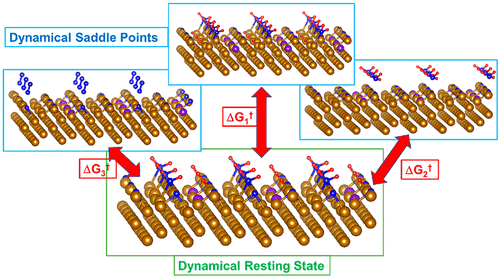
The Haber–Bosch (HB) process is the primary chemical synthesis technique for industrial production of ammonia (NH3) for manufacturing nitrate-based fertilizer and as a potential hydrogen carrier. The HB process alone is responsible for over 2% of all global energy usage to produce more than 160 million tons of NH3 annually. Iron catalysts are utilized to accelerate the reaction, but high temperatures and pressures of atmospheric nitrogen gas (N2) and hydrogen gas (H2) are required. A great deal of research has aimed at increased performance over the last century, but the rate of progress has been slow. This Account focuses on determining the atomic-level reaction mechanism for HB synthesis of NH3 on the Fe catalysts used in industry and how to use this knowledge to suggest greatly improved catalysts via a novel paradigm of catalyst rational design.
中文翻译:

分级高通量催化剂设计的氨合成反应机理、动力学和改进催化剂
Haber-Bosch (HB) 工艺是工业生产氨 (NH 3 ) 的主要化学合成技术,用于制造硝酸盐基肥料并作为潜在的氢载体。仅 HB 工艺就占全球所有能源使用量的 2% 以上,每年生产超过 1.6 亿吨 NH 3。铁催化剂用于加速反应,但需要大气氮气(N 2)和氢气(H 2)的高温和高压。在上个世纪,大量研究旨在提高性能,但进展速度很慢。该帐户侧重于确定 HB 合成 NH 3的原子级反应机制关于工业中使用的铁催化剂以及如何利用这些知识通过催化剂合理设计的新范式提出大大改进的催化剂。
更新日期:2022-04-06
中文翻译:

分级高通量催化剂设计的氨合成反应机理、动力学和改进催化剂
Haber-Bosch (HB) 工艺是工业生产氨 (NH 3 ) 的主要化学合成技术,用于制造硝酸盐基肥料并作为潜在的氢载体。仅 HB 工艺就占全球所有能源使用量的 2% 以上,每年生产超过 1.6 亿吨 NH 3。铁催化剂用于加速反应,但需要大气氮气(N 2)和氢气(H 2)的高温和高压。在上个世纪,大量研究旨在提高性能,但进展速度很慢。该帐户侧重于确定 HB 合成 NH 3的原子级反应机制关于工业中使用的铁催化剂以及如何利用这些知识通过催化剂合理设计的新范式提出大大改进的催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号