当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High-Yield Synthesis of Enantiopure 1,2-Amino Alcohols from l-Phenylalanine via Linear and Divergent Enzymatic Cascades
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-03-28 , DOI: 10.1021/acs.oprd.1c00490 Maria L Corrado 1 , Tanja Knaus 1 , Ulrich Schwaneberg 2 , Francesco G Mutti 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-03-28 , DOI: 10.1021/acs.oprd.1c00490 Maria L Corrado 1 , Tanja Knaus 1 , Ulrich Schwaneberg 2 , Francesco G Mutti 1
Affiliation
|
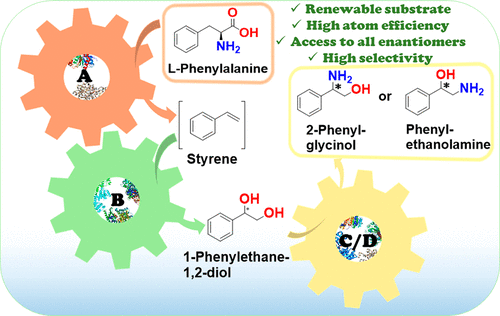
Enantiomerically pure 1,2-amino alcohols are important compounds due to their biological activities and wide applications in chemical synthesis. In this work, we present two multienzyme pathways for the conversion of l-phenylalanine into either 2-phenylglycinol or phenylethanolamine in the enantiomerically pure form. Both pathways start with the two-pot sequential four-step conversion of l-phenylalanine into styrene via subsequent deamination, decarboxylation, enantioselective epoxidation, and enantioselective hydrolysis. For instance, after optimization, the multienzyme process could convert 507 mg of l-phenylalanine into (R)-1-phenyl-1,2-diol in an overall isolated yield of 75% and >99% ee. The opposite enantiomer, (S)-1-phenyl-1,2-diol, was also obtained in a 70% yield and 98–99% ee following the same approach. At this stage, two divergent routes were developed to convert the chiral diols into either 2-phenylglycinol or phenylethanolamine. The former route consisted of a one-pot concurrent interconnected two-step cascade in which the diol intermediate was oxidized to 2-hydroxy-acetophenone by an alcohol dehydrogenase and then aminated by a transaminase to give enantiomerically pure 2-phenylglycinol. Notably, the addition of an alanine dehydrogenase enabled the connection of the two steps and made the overall process redox-self-sufficient. Thus, (S)-phenylglycinol was isolated in an 81% yield and >99.4% ee starting from ca. 100 mg of the diol intermediate. The second route consisted of a one-pot concurrent two-step cascade in which the oxidative and reductive steps were not interconnected. In this case, the diol intermediate was oxidized to either (S)- or (R)-2-hydroxy-2-phenylacetaldehyde by an alcohol oxidase and then aminated by an amine dehydrogenase to give the enantiomerically pure phenylethanolamine. The addition of a formate dehydrogenase and sodium formate was required to provide the reducing equivalents for the reductive amination step. Thus, (R)-phenylethanolamine was isolated in a 92% yield and >99.9% ee starting from ca. 100 mg of the diol intermediate. In summary, l-phenylalanine was converted into enantiomerically pure 2-phenylglycinol and phenylethanolamine in overall yields of 61% and 69%, respectively. This work exemplifies how linear and divergent enzyme cascades can enable the synthesis of high-value chiral molecules such as amino alcohols from a renewable material such as l-phenylalanine with high atom economy and improved sustainability.
中文翻译:

通过线性和发散酶级联从 l-苯丙氨酸高产率合成对映体纯 1,2-氨基醇
对映体纯的 1,2-氨基醇因其生物活性和在化学合成中的广泛应用而成为重要的化合物。在这项工作中,我们提出了两种多酶途径,用于将l-苯丙氨酸转化为对映体纯形式的 2-苯基甘氨醇或苯乙醇胺。两种途径都从L-苯丙氨酸通过随后的脱氨、脱羧、对映选择性环氧化和对映选择性水解的两锅顺序四步转化为苯乙烯开始。例如,经过优化后,多酶工艺可以将 507 mg 的L-苯丙氨酸转化为 ( R ) -1-苯基-1,2-二醇,总分离产率为 75% 和 >99% ee。相反的对映异构体,(S)-1-phenyl-1,2-diol,也以 70% 的收率和 98-99% 的 ee 遵循相同的方法获得。在这个阶段,开发了两条不同的路线将手性二醇转化为 2-苯基甘氨醇或苯乙醇胺。前一种路线由一锅并发互连的两步级联组成,其中二醇中间体通过醇脱氢酶氧化为 2-羟基-苯乙酮,然后通过转氨酶胺化,得到对映体纯的 2-苯基甘氨醇。值得注意的是,添加丙氨酸脱氢酶使这两个步骤连接起来,并使整个过程氧化还原自给自足。因此, ( S)-苯基甘氨醇以 81% 的收率和 >99.4% ee 从大约 100毫克二醇中间体。第二条路线由一锅并发的两步级联组成,其中氧化和还原步骤不相互连接。在这种情况下,二醇中间体通过醇氧化酶氧化为( S )-或( R )-2-羟基-2-苯乙醛,然后通过胺脱氢酶胺化,得到对映体纯的苯乙醇胺。需要添加甲酸脱氢酶和甲酸钠以提供还原胺化步骤的还原当量。因此,( R )-苯基乙醇胺以 92% 的产率和 >99.9% ee 从大约 100毫克二醇中间体。总之,l-苯丙氨酸转化为对映体纯的 2-苯基甘氨醇和苯乙醇胺,总产率分别为 61% 和 69%。这项工作举例说明了线性和发散的酶级联如何能够从具有高原子经济性和提高可持续性的可再生材料(如l-苯丙氨酸)合成高价值手性分子(如氨基醇) 。
更新日期:2022-03-28
中文翻译:

通过线性和发散酶级联从 l-苯丙氨酸高产率合成对映体纯 1,2-氨基醇
对映体纯的 1,2-氨基醇因其生物活性和在化学合成中的广泛应用而成为重要的化合物。在这项工作中,我们提出了两种多酶途径,用于将l-苯丙氨酸转化为对映体纯形式的 2-苯基甘氨醇或苯乙醇胺。两种途径都从L-苯丙氨酸通过随后的脱氨、脱羧、对映选择性环氧化和对映选择性水解的两锅顺序四步转化为苯乙烯开始。例如,经过优化后,多酶工艺可以将 507 mg 的L-苯丙氨酸转化为 ( R ) -1-苯基-1,2-二醇,总分离产率为 75% 和 >99% ee。相反的对映异构体,(S)-1-phenyl-1,2-diol,也以 70% 的收率和 98-99% 的 ee 遵循相同的方法获得。在这个阶段,开发了两条不同的路线将手性二醇转化为 2-苯基甘氨醇或苯乙醇胺。前一种路线由一锅并发互连的两步级联组成,其中二醇中间体通过醇脱氢酶氧化为 2-羟基-苯乙酮,然后通过转氨酶胺化,得到对映体纯的 2-苯基甘氨醇。值得注意的是,添加丙氨酸脱氢酶使这两个步骤连接起来,并使整个过程氧化还原自给自足。因此, ( S)-苯基甘氨醇以 81% 的收率和 >99.4% ee 从大约 100毫克二醇中间体。第二条路线由一锅并发的两步级联组成,其中氧化和还原步骤不相互连接。在这种情况下,二醇中间体通过醇氧化酶氧化为( S )-或( R )-2-羟基-2-苯乙醛,然后通过胺脱氢酶胺化,得到对映体纯的苯乙醇胺。需要添加甲酸脱氢酶和甲酸钠以提供还原胺化步骤的还原当量。因此,( R )-苯基乙醇胺以 92% 的产率和 >99.9% ee 从大约 100毫克二醇中间体。总之,l-苯丙氨酸转化为对映体纯的 2-苯基甘氨醇和苯乙醇胺,总产率分别为 61% 和 69%。这项工作举例说明了线性和发散的酶级联如何能够从具有高原子经济性和提高可持续性的可再生材料(如l-苯丙氨酸)合成高价值手性分子(如氨基醇) 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号