当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis Optimization, Scale-Up, and Catalyst Screening Efforts toward the MGAT2 Clinical Candidate, BMS-963272
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-03-24 , DOI: 10.1021/acs.oprd.2c00036 James Kempson 1 , Xiaoping Hou 1 , Jung-Hui Sun 1 , Michael Wong 1 , Joseph Pawluczyk 1 , Jianqing Li 1 , Subramaniam Krishnananthan 1 , Eric M. Simmons 2 , Yi Hsiao 2 , Yi-Xin Li 1 , Dawn Sun 1 , Dauh-Rurng Wu 1 , Wei Meng 1 , Saleem Ahmad 1 , Lidet Negash 1 , Robert Brigance 1 , Huji Turdi 1 , Jon J. Hangeland 1 , R. Michael Lawrence 1 , Pratik Devasthale 1 , Jeffrey A. Robl 1 , Arvind Mathur 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-03-24 , DOI: 10.1021/acs.oprd.2c00036 James Kempson 1 , Xiaoping Hou 1 , Jung-Hui Sun 1 , Michael Wong 1 , Joseph Pawluczyk 1 , Jianqing Li 1 , Subramaniam Krishnananthan 1 , Eric M. Simmons 2 , Yi Hsiao 2 , Yi-Xin Li 1 , Dawn Sun 1 , Dauh-Rurng Wu 1 , Wei Meng 1 , Saleem Ahmad 1 , Lidet Negash 1 , Robert Brigance 1 , Huji Turdi 1 , Jon J. Hangeland 1 , R. Michael Lawrence 1 , Pratik Devasthale 1 , Jeffrey A. Robl 1 , Arvind Mathur 1
Affiliation
|
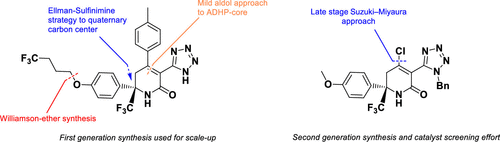
This paper describes the efficient scale-up synthesis of 1 (BMS-963272) which relies upon a highly selective Mannich-type alkylation strategy to stereospecifically install a quaternary carbon center. An intramolecular cyclization reaction is also used to form the aryl dihydropyridone (ADHP) core. The optimized route has been demonstrated to provide more than 100 g of active pharmaceutical ingredient for preclinical toxicology evaluation. A catalyst screening effort is also discussed as part of a complimentary convergent approach which will facilitate a more expedient assessment of back-up molecules bearing aryl diversity at the C4-position of the ADHP core.
中文翻译:

MGAT2 临床候选药物 BMS-963272 的合成优化、放大和催化剂筛选工作
本文描述了1 (BMS-963272) 的有效放大合成,该合成依赖于高选择性曼尼希型烷基化策略以立体定向安装季碳中心。分子内环化反应也用于形成芳基二氢吡啶酮 (ADHP) 核心。已证明优化的路线可为临床前毒理学评估提供超过 100 g 的活性药物成分。作为互补收敛方法的一部分,还讨论了催化剂筛选工作,这将有助于更方便地评估在 ADHP 核心的 C4 位具有芳基多样性的备用分子。
更新日期:2022-03-24
中文翻译:

MGAT2 临床候选药物 BMS-963272 的合成优化、放大和催化剂筛选工作
本文描述了1 (BMS-963272) 的有效放大合成,该合成依赖于高选择性曼尼希型烷基化策略以立体定向安装季碳中心。分子内环化反应也用于形成芳基二氢吡啶酮 (ADHP) 核心。已证明优化的路线可为临床前毒理学评估提供超过 100 g 的活性药物成分。作为互补收敛方法的一部分,还讨论了催化剂筛选工作,这将有助于更方便地评估在 ADHP 核心的 C4 位具有芳基多样性的备用分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号