当前位置:
X-MOL 学术
›
J. Surfactants Deterg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Taguchi design approach for the enhancement of a detergent-biocompatible alkaline thermostable protease production by Streptomyces mutabilis strain TN-X30
Journal of Surfactants and Detergents ( IF 1.6 ) Pub Date : 2022-02-09 , DOI: 10.1002/jsde.12583 Sondes Mechri 1 , Khelifa Bouacem 2, 3 , Taha‐Bilel Chalbi 1 , Marwa Khaled 1 , Fawzi Allala 2 , Amel Bouanane‐Darenfed 2 , Hocine Hacene 2 , Bassem Jaouadi 1
Journal of Surfactants and Detergents ( IF 1.6 ) Pub Date : 2022-02-09 , DOI: 10.1002/jsde.12583 Sondes Mechri 1 , Khelifa Bouacem 2, 3 , Taha‐Bilel Chalbi 1 , Marwa Khaled 1 , Fawzi Allala 2 , Amel Bouanane‐Darenfed 2 , Hocine Hacene 2 , Bassem Jaouadi 1
Affiliation
|
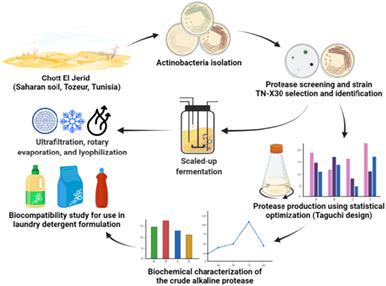
The ability of microorganisms to grow at high temperature, alkaline pH, and high salinity makes them an attractive target for enzyme-production with several industrial applications. One strain TN-X30 has been selected as protease producer and identified as Streptomyces mutabilis after a phenotypic and molecular study. Its production of protease was improved using Taguchi L27 design. The strategy was carried out to identify the optimum levels and the interaction of the screened factors. Following this step, maximum protease activity (10,895 U/ml) was achieved after 6-days of incubation. The TN-X30 protease activity had an optimum of pH and temperature of 10 and 65°C, respectively. Thermodynamic parameters at 60°C were enthalpy 14.26 kJ/mol, entropy −220 J/mol/K, and Gibbs free energy 90.53 kJ/mol. TN-X30 protease production displayed a 16-fold increase reaching 175,000 U/ml in a 100-L fermentor. Furthermore, the lyophilization in presence of sorbitol enhanced the stability of the TN-X30 protease which remained active at 75% after 24-months of storage. The lyophilized TN-X30 protease exhibited exceptional stability indexes in presence of some known commercialized detergent components as NEODOL® 25-7, Dehydol® LT 7, Na2 CMC, Galaxy LAS, Galaxy LES 70, Galaxy 110, Galaxy CAPB Plus, and Sulfacid K. The lyophilized enzyme also displayed high stability with respect to both solid and liquid detergents. Finally, TN-X30 protease exhibited remarkable destaining of blood, egg, and chocolate stained cloth pieces. These findings may promote TN-X30 protease for use as bioadditive in detergent formulation, thereby reducing environmental chemical threat.
中文翻译:

一种用于增强变异链霉菌 TN-X30 产生洗涤剂生物相容性碱性热稳定蛋白酶的田口设计方法
微生物在高温、碱性 pH 值和高盐度下生长的能力使其成为具有多种工业应用的酶生产的有吸引力的目标。一株 TN-X30 已被选为蛋白酶生产者,并被鉴定为Streptomyces mutabilis经过表型和分子研究。使用田口 L27 设计改进了其蛋白酶的生产。执行该策略以确定最佳水平和筛选因素的相互作用。在此步骤之后,孵育 6 天后达到最大蛋白酶活性 (10,895 U/ml)。TN-X30 蛋白酶活性的最佳 pH 值和温度分别为 10 和 65°C。60°C 时的热力学参数为焓 14.26 kJ/mol、熵 -220 J/mol/K 和吉布斯自由能 90.53 kJ/mol。在 100-L 发酵罐中,TN-X30 蛋白酶产量增加了 16 倍,达到 175,000 U/ml。此外,在山梨糖醇存在下的冻干提高了 TN-X30 蛋白酶的稳定性,该蛋白酶在储存 24 个月后仍保持 75% 的活性。2 CMC、Galaxy LAS、Galaxy LES 70、Galaxy 110、Galaxy CAPB Plus 和 Sulfacid K。冻干酶对固体和液体洗涤剂也表现出高稳定性。最后,TN-X30 蛋白酶表现出显着的血液、鸡蛋和巧克力染色布片脱色。这些发现可能会促进 TN-X30 蛋白酶在洗涤剂配方中用作生物添加剂,从而减少环境化学威胁。
更新日期:2022-02-09
中文翻译:

一种用于增强变异链霉菌 TN-X30 产生洗涤剂生物相容性碱性热稳定蛋白酶的田口设计方法
微生物在高温、碱性 pH 值和高盐度下生长的能力使其成为具有多种工业应用的酶生产的有吸引力的目标。一株 TN-X30 已被选为蛋白酶生产者,并被鉴定为Streptomyces mutabilis经过表型和分子研究。使用田口 L27 设计改进了其蛋白酶的生产。执行该策略以确定最佳水平和筛选因素的相互作用。在此步骤之后,孵育 6 天后达到最大蛋白酶活性 (10,895 U/ml)。TN-X30 蛋白酶活性的最佳 pH 值和温度分别为 10 和 65°C。60°C 时的热力学参数为焓 14.26 kJ/mol、熵 -220 J/mol/K 和吉布斯自由能 90.53 kJ/mol。在 100-L 发酵罐中,TN-X30 蛋白酶产量增加了 16 倍,达到 175,000 U/ml。此外,在山梨糖醇存在下的冻干提高了 TN-X30 蛋白酶的稳定性,该蛋白酶在储存 24 个月后仍保持 75% 的活性。2 CMC、Galaxy LAS、Galaxy LES 70、Galaxy 110、Galaxy CAPB Plus 和 Sulfacid K。冻干酶对固体和液体洗涤剂也表现出高稳定性。最后,TN-X30 蛋白酶表现出显着的血液、鸡蛋和巧克力染色布片脱色。这些发现可能会促进 TN-X30 蛋白酶在洗涤剂配方中用作生物添加剂,从而减少环境化学威胁。


























 京公网安备 11010802027423号
京公网安备 11010802027423号