当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Overcoming Acidic H2O2/Fe(II/III) Redox-Induced Low H2O2 Utilization Efficiency by Carbon Quantum Dots Fenton-like Catalysis
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-01-30 , DOI: 10.1021/acs.est.1c06276 Ting Zhang 1 , Yichan Wen 1 , Zhelun Pan 1 , Yasutaka Kuwahara 2 , Kohsuke Mori 2 , Hiromi Yamashita 2 , Yixin Zhao 1, 3 , Xufang Qian 1, 3
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-01-30 , DOI: 10.1021/acs.est.1c06276 Ting Zhang 1 , Yichan Wen 1 , Zhelun Pan 1 , Yasutaka Kuwahara 2 , Kohsuke Mori 2 , Hiromi Yamashita 2 , Yixin Zhao 1, 3 , Xufang Qian 1, 3
Affiliation
|
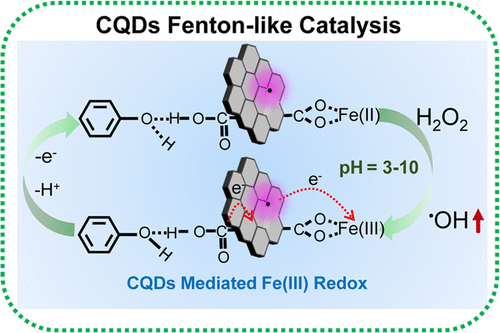
Fenton reaction has important implications in biology- and environment-related remediation. Hydroxyl radicals (•OH) and hydroxide (OH–) were formed by a reaction between Fe(II) and hydrogen peroxide (H2O2). The acidic H2O2/Fe(II/III) redox-induced low H2O2 utilization efficiency is the bottleneck of Fenton reaction. Electron paramagnetic resonance, surface-enhanced Raman scattering, and density functional theory calculation indicate that the unpaired electrons in the defects of carbon quantum dots (CQDs) and the carboxylic groups at the edge have a synergistic effect on CQDs Fenton-like catalysis. This leads to a 33-fold higher H2O2 utilization efficiency in comparison with Fe(II)/H2O2 Fenton reaction, and the pseudo-first-order reaction rate constant (kobs) increases 38-fold that of Fe(III)/H2O2 under equivalent conditions. The replacement of acidic H2O2/Fe(II/III) redox with CQD-mediated Fe(II/III) redox improves the sluggish Fe(II) generation. Highly effective production of •OH in CQDs-Fe(III)/H2O2 dramatically decreases the selectivity of toxic intermediate benzoquinone. The inorganic ions and dissolved organic matter (DOM) in real groundwater show negligible effects on the CQDs Fenton-like catalysis process. This work presents a process with a higher efficiency of utilization of H2O2in situ chemical oxidation (ISCO) to remove persistent organic pollutants.
中文翻译:

通过碳量子点类芬顿催化克服酸性 H2O2/Fe(II/III) 氧化还原引起的 H2O2 低利用效率
Fenton 反应在生物学和环境相关的修复中具有重要意义。羟基自由基 ( • OH) 和氢氧化物 (OH – ) 由 Fe(II) 和过氧化氢 (H 2 O 2 ) 之间的反应形成。酸性H 2 O 2 /Fe(II/III)氧化还原导致H 2 O 2利用率低是Fenton反应的瓶颈。电子顺磁共振、表面增强拉曼散射和密度泛函理论计算表明,碳量子点(CQDs)缺陷中的未配对电子与边缘羧基对CQDs类芬顿催化具有协同作用。这导致 H 高出 33 倍2 O 2利用效率与Fe(II)/H 2 O 2 Fenton反应相比,准一级反应速率常数( k obs )是Fe(III)/H 2 O 2的38倍同等条件。用 CQD 介导的 Fe(II/III) 氧化还原代替酸性 H 2 O 2 /Fe(II/III) 氧化还原改善了缓慢的 Fe(II) 生成。在 CQDs-Fe(III)/H 2 O 2中高效生成• OH大大降低了有毒中间体苯醌的选择性。实际地下水中的无机离子和溶解有机物 (DOM) 对 CQDs 类 Fenton 催化过程的影响可以忽略不计。这项工作提出了一种利用 H 2 O 2原位化学氧化 (ISCO) 去除持久性有机污染物的效率更高的工艺。
更新日期:2022-01-30
中文翻译:

通过碳量子点类芬顿催化克服酸性 H2O2/Fe(II/III) 氧化还原引起的 H2O2 低利用效率
Fenton 反应在生物学和环境相关的修复中具有重要意义。羟基自由基 ( • OH) 和氢氧化物 (OH – ) 由 Fe(II) 和过氧化氢 (H 2 O 2 ) 之间的反应形成。酸性H 2 O 2 /Fe(II/III)氧化还原导致H 2 O 2利用率低是Fenton反应的瓶颈。电子顺磁共振、表面增强拉曼散射和密度泛函理论计算表明,碳量子点(CQDs)缺陷中的未配对电子与边缘羧基对CQDs类芬顿催化具有协同作用。这导致 H 高出 33 倍2 O 2利用效率与Fe(II)/H 2 O 2 Fenton反应相比,准一级反应速率常数( k obs )是Fe(III)/H 2 O 2的38倍同等条件。用 CQD 介导的 Fe(II/III) 氧化还原代替酸性 H 2 O 2 /Fe(II/III) 氧化还原改善了缓慢的 Fe(II) 生成。在 CQDs-Fe(III)/H 2 O 2中高效生成• OH大大降低了有毒中间体苯醌的选择性。实际地下水中的无机离子和溶解有机物 (DOM) 对 CQDs 类 Fenton 催化过程的影响可以忽略不计。这项工作提出了一种利用 H 2 O 2原位化学氧化 (ISCO) 去除持久性有机污染物的效率更高的工艺。

























 京公网安备 11010802027423号
京公网安备 11010802027423号