Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-01-21 , DOI: 10.1016/j.jhep.2021.12.036 Erica L Buonomo 1 , Shenglin Mei 2 , Samantha R Guinn 3 , Isabelle R Leo 4 , Michael J Peluso 1 , Mei-An Nolan 1 , Frank A Schildberg 5 , Lei Zhao 6 , Christine Lian 6 , Shuyun Xu 6 , Joseph Misdraji 7 , Peter V Kharchenko 8 , Arlene H Sharpe 1
|
Background & Aims
Myeloid cells are key regulators of cirrhosis, a major cause of mortality worldwide. Because stromal cells can modulate the functionality of myeloid cells in vitro, targeting stromal-myeloid interactions has become an attractive potential therapeutic strategy. We aimed to investigate how human liver stromal cells impact myeloid cell properties and to understand the utility of a stromal-myeloid coculture system to study these interactions in the context of cirrhosis.
Methods
Single-cell RNA-sequencing analyses of non-cirrhotic (n = 7) and cirrhotic (n = 5) human liver tissue were correlated to the bulk RNA-sequencing results of in vitro cocultured human CD14+ and primary liver stromal cells. Complimentary mechanistic experiments and flow cytometric analysis were performed on human liver stromal-myeloid coculture systems.
Results
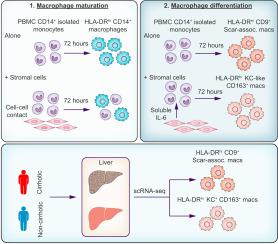
We found that stromal-myeloid coculture reduces the frequency CD14+ cell subsets transcriptionally similar to liver macrophages, showing that stromal cells inhibit the maturation of monocytes into macrophages. Stromal cells also influenced in vitro macrophage differentiation by skewing away from cirrhosis-linked CD9+ scar-associated macrophage-like cells and towards CD163+ Kupffer cell-like macrophages. We identify IL-6 production as a mechanism by which stromal cells limit CD9+ macrophage differentiation and find that local IL-6 levels are decreased in early-stage human liver disease compared to healthy liver tissue, suggesting a protective role for local IL-6 in the healthy liver.
Conclusions
Our work reveals an unanticipated role for liver stromal cells in impeding the maturation and altering the differentiation of macrophages and should prompt investigations into the role of local IL-6 production in the pathogenesis of liver disease. These studies provide a framework for investigating macrophage-stromal interactions during cirrhosis.
Lay summary
The impact of human liver stromal cells on myeloid cell maturation and differentiation in liver disease is incompletely understood. In this study, we present a mechanistic analysis using a primary in vitro human liver stromal-myeloid coculture system that is translated to liver disease using single-cell RNA sequencing analysis of cirrhotic and non-cirrhotic human liver tissue. Our work supports a role for stromal cell contact in restricting macrophage maturation and for stromal-derived IL-6 in limiting the differentiation of a cirrhotic macrophage subset.
中文翻译:

肝基质细胞限制巨噬细胞成熟,基质 IL-6 限制肝硬化相关巨噬细胞的分化
背景与目标
髓细胞是肝硬化的关键调节因子,肝硬化是全世界死亡的主要原因。由于基质细胞可以在体外调节骨髓细胞的功能,因此靶向基质-骨髓相互作用已成为一种有吸引力的潜在治疗策略。我们旨在研究人类肝脏基质细胞如何影响骨髓细胞特性,并了解基质-骨髓共培养系统在肝硬化背景下研究这些相互作用的效用。
方法
非肝硬化 (n = 7) 和肝硬化 (n = 5) 人肝组织的单细胞 RNA 测序分析与体外共培养的人 CD14 +和原代肝基质细胞的大量 RNA 测序结果相关。在人肝基质-骨髓共培养系统上进行了免费的机械实验和流式细胞仪分析。
结果
我们发现基质-骨髓共培养降低了与肝巨噬细胞转录相似的 CD14 +细胞亚群的频率,表明基质细胞抑制单核细胞成熟为巨噬细胞。基质细胞还通过从与肝硬化相关的 CD9 +瘢痕相关的巨噬细胞样细胞向 CD163 +枯否细胞样巨噬细胞倾斜来影响体外巨噬细胞分化。我们将 IL-6 的产生确定为基质细胞限制 CD9 +巨噬细胞分化的机制,并发现与健康肝组织相比,早期人类肝病的局部 IL-6 水平降低,表明局部 IL-6 具有保护作用在健康的肝脏中。
结论
我们的工作揭示了肝基质细胞在阻碍巨噬细胞成熟和改变分化方面的意想不到的作用,并应促进对局部 IL-6 产生在肝病发病机制中的作用的调查。这些研究为研究肝硬化期间的巨噬细胞-基质相互作用提供了一个框架。
总结
人类肝脏基质细胞对肝脏疾病中骨髓细胞成熟和分化的影响尚不完全清楚。在这项研究中,我们使用原发性体外人肝基质-骨髓共培养系统进行机械分析,该系统使用肝硬化和非肝硬化人肝组织的单细胞 RNA 测序分析转化为肝病。我们的工作支持基质细胞接触在限制巨噬细胞成熟和基质衍生的 IL-6 在限制肝硬化巨噬细胞亚群分化中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号