当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determination and Correlation of Phase Equilibrium of the Ethanol/Isopropanol + Manganese Sulfate + Water Ternary Systems
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jced.1c00777 Hua Yang 1 , Jun Li 1 , Yang Jin 1 , Ming Chen 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jced.1c00777 Hua Yang 1 , Jun Li 1 , Yang Jin 1 , Ming Chen 1
Affiliation
|
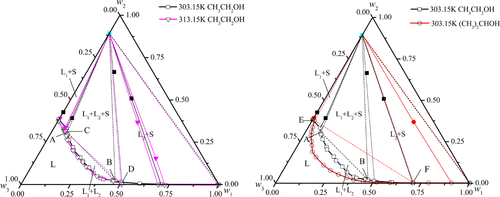
The phase equilibrium of MnSO4 in water-alcohol solution is indispensable for the separation and purification of MnSO4 based on solvent-out effects. To investigate the phase behavior of MnSO4 in water-alcohol solution, the phase equilibrium of CH3CH2OH + MnSO4 + H2O systems at 303.15 and 313.15 K and the (CH3)2CHOH + MnSO4 + H2O system at 303.15 K has been determined. Five regions, namely, L (liquid), L1 + S (liquid 1 + solid), L1 + L2 (liquid 1 + liquid 2), L1 + L2 + S (liquid 1 + liquid 2 + solid), and L2 + S (liquid 2 + solid), are observed. Furthermore, the effects of alcohol types and temperatures on the formation of aqueous two-phase systems (ATPSs) are discussed in detail. It is found that the three experimental systems are partially miscible systems. The (CH3)2CHOH + MnSO4 + H2O system has a larger split phase region and better solvent-out effect than the CH3CH2OH + MnSO4 + H2O systems. Meanwhile, the temperature has a slight influence on the phase behavior and the formation of ATPSs. The solubility of MnSO4 decreases with an increasing alcohol concentration. In addition, the relationship between the w(MnSO4) and w(alcohols) of the binodal curves is obtained based on the Mistry equation, Merchuk equation, and Pirdashti equation. The NRTL model is applied to fit the liquid–liquid equilibrium. The correlation coefficients show that the experimental data is in good agreement with the calculated values. It is worth mentioning that the equilibrium solid phase is MnSO4·H2O. This work provides basic data for manganese sulfate purification and can be applied to develop thermodynamic models of ATPSs.
中文翻译:

乙醇/异丙醇+硫酸锰+水三元体系相平衡的测定及相关性
MnSO 4在水-醇溶液中的相平衡对于基于溶出效应的MnSO 4的分离和纯化是必不可少的。为了研究 MnSO 4在水-醇溶液中的相行为,CH 3 CH 2 OH + MnSO 4 + H 2 O 体系在 303.15 和 313.15 K 和 (CH 3 ) 2 CHOH + MnSO 4 + H 2的相平衡已确定 303.15 K 的 O 系统。五个区域,即L(液体)、L 1 + S(液体1+固体)、L 1 + L 2(液体1+液体2)、L 1观察到+L 2 + S(液体1+液体2+固体)和L 2 + S(液体2+固体)。此外,还详细讨论了醇类型和温度对水性两相系统 (ATPS) 形成的影响。发现三个实验体系是部分混溶体系。(CH 3 ) 2 CHOH + MnSO 4 + H 2 O体系比CH 3 CH 2 OH + MnSO 4 + H 2 O体系具有更大的分相区和更好的溶剂去除效果。同时,温度对相行为和ATPSs的形成有轻微影响。MnSO 4的溶解度随着酒精浓度的增加而减少。另外,基于Mistry方程、Merchuk方程和Pirdashti方程,得到双节曲线的w (MnSO 4 )和w (醇)之间的关系。NRTL 模型用于拟合液-液平衡。相关系数表明,实验数据与计算值吻合较好。值得一提的是,平衡固相为MnSO 4 ·H 2 O。这项工作为硫酸锰提纯提供了基础数据,可用于开发ATPSs的热力学模型。
更新日期:2022-02-10
中文翻译:

乙醇/异丙醇+硫酸锰+水三元体系相平衡的测定及相关性
MnSO 4在水-醇溶液中的相平衡对于基于溶出效应的MnSO 4的分离和纯化是必不可少的。为了研究 MnSO 4在水-醇溶液中的相行为,CH 3 CH 2 OH + MnSO 4 + H 2 O 体系在 303.15 和 313.15 K 和 (CH 3 ) 2 CHOH + MnSO 4 + H 2的相平衡已确定 303.15 K 的 O 系统。五个区域,即L(液体)、L 1 + S(液体1+固体)、L 1 + L 2(液体1+液体2)、L 1观察到+L 2 + S(液体1+液体2+固体)和L 2 + S(液体2+固体)。此外,还详细讨论了醇类型和温度对水性两相系统 (ATPS) 形成的影响。发现三个实验体系是部分混溶体系。(CH 3 ) 2 CHOH + MnSO 4 + H 2 O体系比CH 3 CH 2 OH + MnSO 4 + H 2 O体系具有更大的分相区和更好的溶剂去除效果。同时,温度对相行为和ATPSs的形成有轻微影响。MnSO 4的溶解度随着酒精浓度的增加而减少。另外,基于Mistry方程、Merchuk方程和Pirdashti方程,得到双节曲线的w (MnSO 4 )和w (醇)之间的关系。NRTL 模型用于拟合液-液平衡。相关系数表明,实验数据与计算值吻合较好。值得一提的是,平衡固相为MnSO 4 ·H 2 O。这项工作为硫酸锰提纯提供了基础数据,可用于开发ATPSs的热力学模型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号