Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-01-20 , DOI: 10.1016/j.jhep.2021.12.041 Simon Pape 1 , Romée J A L M Snijders 1 , Tom J G Gevers 2 , Oliver Chazouilleres 3 , George N Dalekos 4 , Gideon M Hirschfield 5 , Marco Lenzi 6 , Michael Trauner 7 , Michael P Manns 8 , John M Vierling 9 , Aldo J Montano-Loza 10 , Ansgar W Lohse 11 , Christoph Schramm 12 , Joost P H Drenth 1 , Michael A Heneghan 13 ,
|
Background & Aims
Autoimmune hepatitis (AIH) has been well characterised and codified through the development of diagnostic criteria. These criteria have been adapted and simplified and are widely used in clinical practice. However, there is a need to update and precisely define the criteria for both treatment response and treatment.
Methods
A systematic review was performed and a modified Delphi consensus process was used to identify and redefine the response criteria in autoimmune hepatitis.
Results
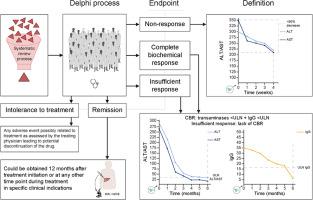
The consensus process initiated by the International Autoimmune Hepatitis Group proposes that the term ‘complete biochemical response’ defined as ‘normalization of serum transaminases and IgG below the upper limit of normal’ be adopted to include a time point at 6 months after initiation of treatment. An insufficient response by 6 months was a failure to meet the above definition. Non-response was defined as ‘<50% decrease of serum transaminases within 4 weeks after initiation of treatment’. Remission is defined as liver histology with a Hepatitis Activity Index <4/18. Intolerance to treatment was agreed to stand for ‘any adverse event possibly related to treatment leading to potential drug discontinuation’.
Conclusions
These definitions provide a simple and reproducible framework to define treatment response and non-response, irrespective of the therapeutic intervention. A consensus on endpoints is urgently required to set a global standard for the reporting of study results and to enable inter-study comparisons. Future prospective database studies are needed to validate these endpoints.
Lay summary
Consensus among international experts on response criteria and endpoints in autoimmune hepatitis is lacking. A consensus on endpoints is urgently required to set a global standard for the reporting of study results and to enable the comparison of results between clinical trials. Therefore, the International Autoimmune Hepatitis Group (IAIHG) herein presents a statement on 5 agreed response criteria and endpoints: complete biochemical response, insufficient response, non-response, remission, and intolerance to treatment, which can be used to guide future reporting.
中文翻译:

国际自身免疫性肝炎小组对自身免疫性肝炎反应标准和终点的系统评价
背景与目标
通过制定诊断标准,自身免疫性肝炎 (AIH) 已得到很好的表征和编纂。这些标准已经过改编和简化,并广泛用于临床实践。然而,需要更新和精确定义治疗反应和治疗的标准。
方法
进行了系统回顾,并使用修改后的德尔菲共识过程来确定和重新定义自身免疫性肝炎的反应标准。
结果
国际自身免疫性肝炎小组发起的共识进程建议采用术语“完全生化反应”定义为“血清转氨酶和 IgG 正常化低于正常上限”,以包括治疗开始后 6 个月的时间点。6 个月后反应不足是不符合上述定义。无反应定义为“治疗开始后 4 周内血清转氨酶降低 <50%”。缓解定义为肝脏组织学肝炎活动指数<4/18。对治疗的不耐受被认为代表“可能与导致潜在药物停药的治疗相关的任何不良事件”。
结论
这些定义提供了一个简单且可重复的框架来定义治疗反应和无反应,与治疗干预无关。迫切需要就终点达成共识,以制定研究结果报告的全球标准,并实现研究间比较。需要未来的前瞻性数据库研究来验证这些终点。
外行总结
国际专家对自身免疫性肝炎的反应标准和终点缺乏共识。迫切需要就终点达成共识,以制定研究结果报告的全球标准,并能够比较临床试验之间的结果。因此,国际自身免疫性肝炎小组 (IAIHG) 在此就 5 个公认的反应标准和终点发表声明:完全生化反应、反应不足、无反应、缓解和对治疗不耐受,可用于指导未来的报告。


























 京公网安备 11010802027423号
京公网安备 11010802027423号