Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2022-01-13 , DOI: 10.1016/j.jece.2022.107192 Alec B. Nienhauser 1 , Mahmut S. Ersan 1 , Zunhui Lin 1 , François Perreault 1 , Paul Westerhoff 1 , Sergi Garcia-Segura 1
|
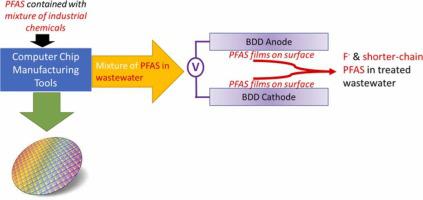
Electrochemical oxidation (ECO) with boron-doped diamond (BDD) electrode of six legacy perfluoroalkyl substances (PFAS) present in semiconductor fabrication wastewaters was investigated in single-solutes, multi-solutes, and real wastewater matrices. ECO degradation kinetics decreased as carbon length decreased in single-solute systems. Because heterogeneous catalysis involves PFAS interacting at the electrode surface, the impact of PFAS in solution on altering the potential affinity for PFAS itself on the surface was quantified. The BDD electrode surface wettability, defined by the surface contact angle, increased with longer chain length and diminished with increasing PFAS concentration. PFASs with a sulfonated head group degraded 2–3x faster than their carboxylic counterparts. Industrial wastewaters can have high background organic concentrations and have a large pH range prior to discharge, offering different opportunities to treat for PFAS near the semiconductor manufacturing tool or prior to pH adjustment and discharge to sanitary sewers. Increasing pH (up to 12) decreased the evolution of reactive oxygen species on the electrode surface and therefore decreased removal kinetics compared to pH 3 and 7. The presence of surfactants (tetramethylammonium hydroxide [TMAH] and sodium dodecyl sulfonate [SDS]) in the wastewater significantly reduced the degradation efficiency of ECO. However, compared to single-solute PFAS systems, multi-solute solutions (mixture of 6 PFASs) had higher degradation rates during ECO. In a real wastewater (originated from RO concentrate), PFASs were efficiently removed in ~320 min reaction time. These results demonstrated the promise for ECO to treat PFAS contained in industrial manufacturing processes at both tool level and blended wastewater.
中文翻译:

掺硼金刚石电极可降解实际工业废水中的短链和长链全氟和多氟烷基物质
在单溶质、多溶质和实际废水基质中研究了半导体制造废水中存在的六种传统全氟烷基物质 (PFAS) 的硼掺杂金刚石 (BDD) 电极的电化学氧化 (ECO)。ECO 降解动力学随着单溶质系统中碳长度的减少而降低。由于多相催化涉及 PFAS 在电极表面的相互作用,因此量化了溶液中 PFAS 对改变 PFAS 本身在表面上的潜在亲和力的影响。由表面接触角定义的 BDD 电极表面润湿性随着链长的增加而增加,随着 PFAS 浓度的增加而减少。具有磺化头基的 PFAS 的降解速度比其羧基对应物快 2-3 倍。工业废水在排放前可能具有高背景有机物浓度和较大的 pH 值范围,这为在半导体制造工具附近或在调整 pH 值和排放到下水道之前处理 PFAS 提供了不同的机会。与 pH 值 3 和 7 相比,增加 pH 值(最高 12)会减少电极表面活性氧物质的释放,因此会降低去除动力学。表面活性剂(四甲基氢氧化铵 [TMAH] 和十二烷基磺酸钠 [SDS])的存在废水显着降低了ECO的降解效率。然而,与单溶质 PFAS 系统相比,多溶质溶液(6 种 PFAS 的混合物)在 ECO 期间具有更高的降解率。在真正的废水(源自 RO 浓缩液)中,PFAS 在约 320 分钟的反应时间内被有效去除。


























 京公网安备 11010802027423号
京公网安备 11010802027423号