当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isothermal Vapor–Liquid Equilibrium (P–T–x–y) Measurements and Modeling of Propan-2-ol + n-Octane/n-Decane in the Range of T = 313.2–353.2 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-11 , DOI: 10.1021/acs.jced.1c00794 Kuveneshan Moodley 1 , Shivan Mavalal 1 , Thavashni Chetty 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-01-11 , DOI: 10.1021/acs.jced.1c00794 Kuveneshan Moodley 1 , Shivan Mavalal 1 , Thavashni Chetty 1
Affiliation
|
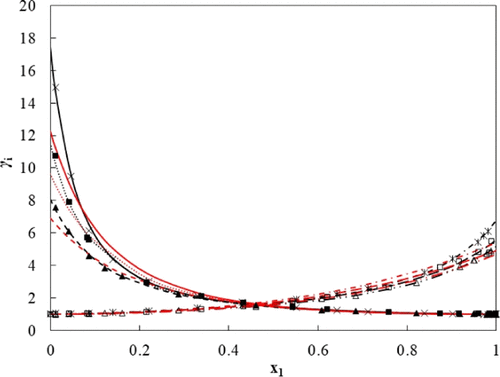
Vapor–liquid equilibrium measurements (P–T–x–y) are presented for propan-2-ol + n-octane/n-decane mixtures at three temperatures (T = 313.2, 333.2, and 353.2 K) and sub-atmospheric pressures, which were determined by the dynamic method in a recirculating still. The systems exhibit large relative volatilities, with azeotropic behavior observed for the propan-2-ol + n-octane system at all three measured temperatures. The experimental data were modeled using the γ–Φ regression formulation with the non-random two-liquid or UNIQUAC activity coefficient models implemented to describe the non-ideality of the liquid phase and the Hayden and O’Connell correlation in the virial equation of state to describe the non-ideality of the vapor phase. The non-random two-liquid model outperformed the UNIQUAC model in fitting the experimental data. The experimental data were found to be thermodynamically consistent using the area, point, and infinite dilution tests.
中文翻译:

T = 313.2–353.2 K 范围内的 Propan-2-ol + n-Octane/n-Decane 的等温汽液平衡 (P–T–x–y) 测量和建模
对丙-2-醇 +正辛烷/正癸烷混合物在三个温度(T = 313.2、333.2 和 353.2 K)和低于大气压的压力下进行汽液平衡测量 ( P – T – x – y ) , 由循环蒸馏器中的动态方法确定。系统表现出较大的相对挥发性,观察到 propan-2-ol + n的共沸行为-辛烷系统在所有三个测量温度。使用 γ-Φ 回归公式对实验数据进行建模,并使用非随机二液或 UNIQUAC 活度系数模型来描述液相的非理想性以及维里状态方程中的 Hayden 和 O'Connell 相关性来描述气相的非理想性。非随机二液模型在拟合实验数据方面优于 UNIQUAC 模型。使用面积、点和无限稀释试验发现实验数据在热力学上是一致的。
更新日期:2022-02-10
中文翻译:

T = 313.2–353.2 K 范围内的 Propan-2-ol + n-Octane/n-Decane 的等温汽液平衡 (P–T–x–y) 测量和建模
对丙-2-醇 +正辛烷/正癸烷混合物在三个温度(T = 313.2、333.2 和 353.2 K)和低于大气压的压力下进行汽液平衡测量 ( P – T – x – y ) , 由循环蒸馏器中的动态方法确定。系统表现出较大的相对挥发性,观察到 propan-2-ol + n的共沸行为-辛烷系统在所有三个测量温度。使用 γ-Φ 回归公式对实验数据进行建模,并使用非随机二液或 UNIQUAC 活度系数模型来描述液相的非理想性以及维里状态方程中的 Hayden 和 O'Connell 相关性来描述气相的非理想性。非随机二液模型在拟合实验数据方面优于 UNIQUAC 模型。使用面积、点和无限稀释试验发现实验数据在热力学上是一致的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号