当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Nitrophenolic Byproducts during UV-Activated Peroxydisulfate Oxidation in the Presence of Nitrate
ACS ES&T Engineering Pub Date : 2022-01-07 , DOI: 10.1021/acsestengg.1c00356 Xu Gao 1 , Qi Zhang 1 , Ziyi Yang 1 , Yuefei Ji 1 , Jing Chen 1 , Junhe Lu 1
ACS ES&T Engineering Pub Date : 2022-01-07 , DOI: 10.1021/acsestengg.1c00356 Xu Gao 1 , Qi Zhang 1 , Ziyi Yang 1 , Yuefei Ji 1 , Jing Chen 1 , Junhe Lu 1
Affiliation
|
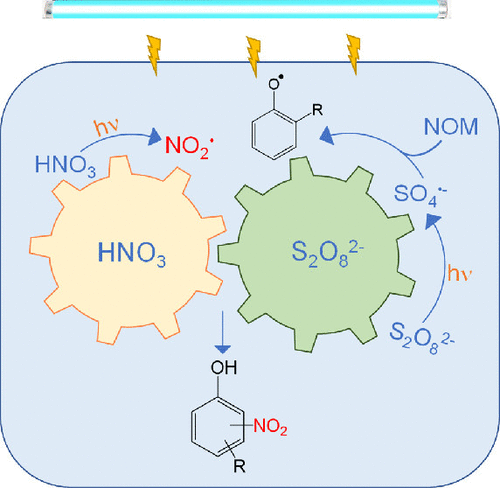
UV-peroxydisulfate (UV/PDS) oxidation is a promising technology to degrade organic pollutants. In this study, we found that nitrate (NO3–) could lead to the formation of toxic nitrophenolic byproducts during UV/PDS oxidation of natural organic matter (NOM). At a UV fluence of 4.49 × 105 mJ L–1, the formation of 4-nitrophenol, 4-hydroxy-3-nitrobenzoic acid, and 2,4-dinitrophenol reached 0.0084, 0.0324, and 0.0046 μM, respectively. NO2• produced from the photolysis of NO3– acted as the nitrating agent. Meanwhile, the phenolic moieties of NOM molecules were oxidized by SO4•– through single-electron transfer, giving rise to phenoxyl radicals. The phenoxyl radicals coupled with NO2• to generate nitrated byproducts. Although •OH was also formed in the UV/PDS process, theoretical computation suggests that the phenoxyl radicals were primarily ascribed to SO4•–, because •OH preferentially reacted with NOM via addition mechanism resulting in hydroxylated intermediates. In addition, the aromatic carboxyl moieties of NOM molecules could be decarboxylated upon reaction with SO4•– and transformed to phenolic intermediates, which also contributed to the nitrated byproducts formation. This study reveals a novel nitration mechanism that is specific to the UV/PDS process and raises concerns to the potential risks when the UV/PDS is applied to wastewaters with high levels of NO3–.
中文翻译:

硝酸盐存在下紫外活化过二硫酸盐氧化过程中硝基酚类副产物的形成
UV-过二硫酸盐(UV/PDS)氧化是一种很有前途的有机污染物降解技术。在这项研究中,我们发现硝酸盐 (NO 3 – ) 会导致在 UV/PDS 氧化天然有机物 (NOM) 过程中形成有毒的硝基酚副产物。在 4.49 × 10 5 mJ L -1的 UV 能量密度下,4-硝基苯酚、4-羟基-3-硝基苯甲酸和 2,4-二硝基苯酚的生成量分别达到 0.0084、0.0324 和 0.0046 μM。NO 2 •由 NO 3光解产生——作为硝化剂。同时,NOM分子的酚类部分被SO 4氧化•–通过单电子转移,产生苯氧基自由基。苯氧基自由基与NO 2 •偶联生成硝化副产物。尽管• OH 也在 UV/PDS 过程中形成,但理论计算表明苯氧基自由基主要归因于 SO 4 •–,因为• OH 通过加成机制优先与 NOM 反应,产生羟基化中间体。此外,NOM 分子的芳族羧基部分可在与 SO 4反应时脱羧•–并转化为酚类中间体,这也有助于硝化副产物的形成。这项研究揭示了一种新的硝化机制,该机制特定于 UV/PDS 工艺,并引起了人们对当 UV/PDS 应用于高浓度 NO 3 -废水时的潜在风险的担忧。
更新日期:2022-02-11
中文翻译:

硝酸盐存在下紫外活化过二硫酸盐氧化过程中硝基酚类副产物的形成
UV-过二硫酸盐(UV/PDS)氧化是一种很有前途的有机污染物降解技术。在这项研究中,我们发现硝酸盐 (NO 3 – ) 会导致在 UV/PDS 氧化天然有机物 (NOM) 过程中形成有毒的硝基酚副产物。在 4.49 × 10 5 mJ L -1的 UV 能量密度下,4-硝基苯酚、4-羟基-3-硝基苯甲酸和 2,4-二硝基苯酚的生成量分别达到 0.0084、0.0324 和 0.0046 μM。NO 2 •由 NO 3光解产生——作为硝化剂。同时,NOM分子的酚类部分被SO 4氧化•–通过单电子转移,产生苯氧基自由基。苯氧基自由基与NO 2 •偶联生成硝化副产物。尽管• OH 也在 UV/PDS 过程中形成,但理论计算表明苯氧基自由基主要归因于 SO 4 •–,因为• OH 通过加成机制优先与 NOM 反应,产生羟基化中间体。此外,NOM 分子的芳族羧基部分可在与 SO 4反应时脱羧•–并转化为酚类中间体,这也有助于硝化副产物的形成。这项研究揭示了一种新的硝化机制,该机制特定于 UV/PDS 工艺,并引起了人们对当 UV/PDS 应用于高浓度 NO 3 -废水时的潜在风险的担忧。


























 京公网安备 11010802027423号
京公网安备 11010802027423号