当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the Critical Role of Ruthenium Single Atom Sites in Water Dissociation and Dehydrogenation Behaviors for Robust Hydrazine Oxidation-Boosted Alkaline Hydrogen Evolution
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2022-01-05 , DOI: 10.1002/adfm.202109439 Jiachen Li 1, 2 , Yang Li 3 , Jiaao Wang 4 , Chi Zhang 1 , Huijun Ma 5 , Chenhui Zhu 3 , Daidi Fan 3 , Zhaoqi Guo 1 , Ming Xu 6 , Yaoyu Wang 2 , Haixia Ma 1
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2022-01-05 , DOI: 10.1002/adfm.202109439 Jiachen Li 1, 2 , Yang Li 3 , Jiaao Wang 4 , Chi Zhang 1 , Huijun Ma 5 , Chenhui Zhu 3 , Daidi Fan 3 , Zhaoqi Guo 1 , Ming Xu 6 , Yaoyu Wang 2 , Haixia Ma 1
Affiliation
|
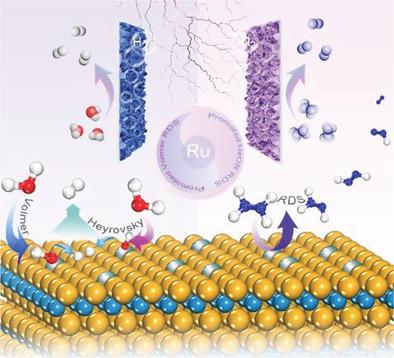
Hydrazine oxidation (HzOR)-assisted overall water splitting (OWS) provides a unique approach to energy-efficient hydrogen production (HER). However, there are still major challenges in the design of bifunctional catalysts and gain deep insight into the mechanism of both water dissociation and dehydrogenation kinetics triggered by the same active species during HzOR-assisted OWS. Here, ruthenium single atoms (Ru SAs) anchored onto sulphur-vacancies of tungsten disulphide (WS2) are prepared by a sulfidation and facile galvanostatic deposition strategy. The WS2/Ru SAs act as a bifunctional catalyst and outperforms commercial platinum (Pt) catalysts for both HzOR and HER. Ultralow potentials of −74 and −32.1 mV at 10 mA cm−2 are achieved for HzOR and HER, respectively. Two-electrode electrolyzer using WS2/Ru SAs as both anode and cathode reaches 10 mA cm−2 with cell voltage of only 15.4 mV, which is far below that of most electrocatalysts including commercial Pt. Density functional theory calculations unravel the critical role of Ru SAs in WS2, where the sluggish dissociation of water in HER can be promoted on Ru sites, and the sulfur sites of WS2 exhibit a more thermoneutral behavior for hydrogen intermediate adsorption. Moreover, Ru sites are also active centers for stepwise hydrazine dehydrogenation during HzOR.
中文翻译:

阐明钌单原子位点在水解离和脱氢行为中的关键作用,以实现稳健的肼氧化促进碱性析氢
肼氧化 (HzOR) 辅助的整体水分解 (OWS) 提供了一种独特的节能制氢 (HER) 方法。然而,在双功能催化剂的设计和深入了解在 HzOR 辅助 OWS 期间由相同活性物质触发的水离解和脱氢动力学机制方面仍然存在重大挑战。在这里,锚定在二硫化钨 (WS 2 ) 的硫空位上的钌单原子 (Ru SAs)是通过硫化和简便的恒电流沉积策略制备的。WS 2 /Ru SAs 作为双功能催化剂,在 HzOR 和 HER 方面都优于商业铂 (Pt) 催化剂。在 10 mA cm -2时 -74 和 -32.1 mV 的超低电位分别针对 HzOR 和 HER 实现。使用 WS 2 /Ru SAs 作为阳极和阴极的双电极电解槽达到 10 mA cm -2,电池电压仅为 15.4 mV,远低于包括商业 Pt 在内的大多数电催化剂。密度泛函理论计算揭示了 Ru SAs 在 WS 2中的关键作用,其中在 Ru 位点上可以促进 HER 中水的缓慢解离,并且 WS 2的硫位点对氢中间体吸附表现出更热中性的行为。此外,Ru位点也是HzOR过程中逐步肼脱氢的活性中心。
更新日期:2022-01-05
中文翻译:

阐明钌单原子位点在水解离和脱氢行为中的关键作用,以实现稳健的肼氧化促进碱性析氢
肼氧化 (HzOR) 辅助的整体水分解 (OWS) 提供了一种独特的节能制氢 (HER) 方法。然而,在双功能催化剂的设计和深入了解在 HzOR 辅助 OWS 期间由相同活性物质触发的水离解和脱氢动力学机制方面仍然存在重大挑战。在这里,锚定在二硫化钨 (WS 2 ) 的硫空位上的钌单原子 (Ru SAs)是通过硫化和简便的恒电流沉积策略制备的。WS 2 /Ru SAs 作为双功能催化剂,在 HzOR 和 HER 方面都优于商业铂 (Pt) 催化剂。在 10 mA cm -2时 -74 和 -32.1 mV 的超低电位分别针对 HzOR 和 HER 实现。使用 WS 2 /Ru SAs 作为阳极和阴极的双电极电解槽达到 10 mA cm -2,电池电压仅为 15.4 mV,远低于包括商业 Pt 在内的大多数电催化剂。密度泛函理论计算揭示了 Ru SAs 在 WS 2中的关键作用,其中在 Ru 位点上可以促进 HER 中水的缓慢解离,并且 WS 2的硫位点对氢中间体吸附表现出更热中性的行为。此外,Ru位点也是HzOR过程中逐步肼脱氢的活性中心。


























 京公网安备 11010802027423号
京公网安备 11010802027423号