当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility and Thermodynamic Properties of Sulfuryl Fluoride in Some Biobased Citrate Derivatives
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-12-29 , DOI: 10.1021/acs.jced.1c00731 Xiaojiang Liang 1 , Zhujiao Zhang 1 , Jiawei Ma 1 , Qinglong Xie 1 , Yong Nie 1 , Jianbing Ji 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-12-29 , DOI: 10.1021/acs.jced.1c00731 Xiaojiang Liang 1 , Zhujiao Zhang 1 , Jiawei Ma 1 , Qinglong Xie 1 , Yong Nie 1 , Jianbing Ji 1
Affiliation
|
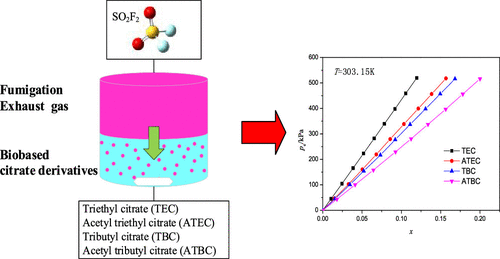
The solubilities of sulfuryl fluoride (SO2F2) in triethyl citrate (TEC), acetyl triethyl citrate (ATEC), tributyl citrate (TBC), and acetyl tributyl citrate (ATBC) were measured using the isochoric saturation method under pressures of up to 600 kPa and at temperatures ranging from 293.15 to 323.15 K. The results show that the SO2F2 absorption in these solvents is a physical process, and the solubility follows the order of ATBC > TBC > ATEC > TEC. Furthermore, Henry’s law constants and thermodynamic properties such as standard Gibbs free energy, enthalpy, and entropy changes of SO2F2 solvation were obtained. Henry’s law constant based on mole fraction in citrate derivatives varies from 2.02 to 5.79 MPa at T = 293.15 to 323.15 K. All the dissolution enthalpies were negative under experimental conditions. Additionally, the performance of SO2F2 in these citrate derivatives was further compared with that in other physical absorbents. It proved that these biobased citrate derivatives have potential use in the removal of SO2F2 from fumigation exhaust gas.
中文翻译:

硫酰氟在一些生物基柠檬酸盐衍生物中的溶解度和热力学性质
使用等容饱和法在最高至600 kPa,温度范围为293.15~323.15 K。结果表明,SO 2 F 2在这些溶剂中的吸收是一个物理过程,溶解度的顺序为ATBC > TBC > ATEC > TEC。此外,亨利定律常数和热力学性质,如标准吉布斯自由能、焓和 SO 2 F 2的熵变得到溶剂化。基于柠檬酸盐衍生物摩尔分数的亨利定律常数在T = 293.15 至 323.15 K 时从 2.02 至 5.79 MPa 变化。在实验条件下,所有溶解焓均为负值。此外,进一步将这些柠檬酸盐衍生物中 SO 2 F 2的性能与其他物理吸收剂中的性能进行了比较。证明这些生物基柠檬酸盐衍生物在熏蒸废气中SO 2 F 2的去除方面具有潜在用途。
更新日期:2022-01-13
中文翻译:

硫酰氟在一些生物基柠檬酸盐衍生物中的溶解度和热力学性质
使用等容饱和法在最高至600 kPa,温度范围为293.15~323.15 K。结果表明,SO 2 F 2在这些溶剂中的吸收是一个物理过程,溶解度的顺序为ATBC > TBC > ATEC > TEC。此外,亨利定律常数和热力学性质,如标准吉布斯自由能、焓和 SO 2 F 2的熵变得到溶剂化。基于柠檬酸盐衍生物摩尔分数的亨利定律常数在T = 293.15 至 323.15 K 时从 2.02 至 5.79 MPa 变化。在实验条件下,所有溶解焓均为负值。此外,进一步将这些柠檬酸盐衍生物中 SO 2 F 2的性能与其他物理吸收剂中的性能进行了比较。证明这些生物基柠檬酸盐衍生物在熏蒸废气中SO 2 F 2的去除方面具有潜在用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号