当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding Solvation Behavior of Cefazolin Sodium in the Aqueous Choline Chloride/Ethylene Glycol or Urea Solutions through Vapor Pressure Osmometry and Volumetric and Acoustic Measurements
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-12-28 , DOI: 10.1021/acs.jced.1c00768 Hemayat Shekaari 1 , Mohammed Taghi Zafarani-Moattar 1 , Masumeh Mokhtarpour 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-12-28 , DOI: 10.1021/acs.jced.1c00768 Hemayat Shekaari 1 , Mohammed Taghi Zafarani-Moattar 1 , Masumeh Mokhtarpour 1
Affiliation
|
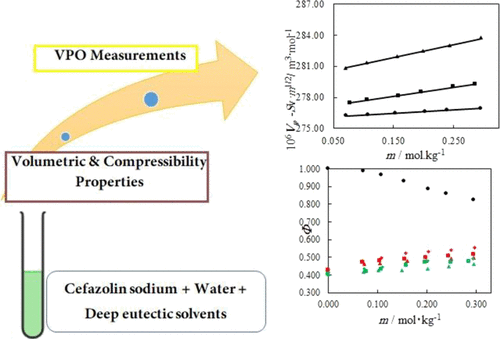
Recently, environmentally green solvents have been indispensable alternatives to traditional organic solvents. Deep eutectic solvents (DESs) have received great attention in numerous uses because of their unique properties. This work was performed within two steps. First, vapor pressure osmometry measurements were applied to determine the osmotic coefficients for binary cefazolin sodium (cefazolin sodium/water) and ternary (cefazolin sodium/DESs: choline chloride/ethylene glycol and choline chloride/urea/water) solutions at T = 298.15 K and p = 872 hPa. For binary solutions, water activity, osmotic coefficients of the solution, and vapor pressure depression were determined. The Pitzer equation was applied to fit the osmotic coefficient data, and from the obtained parameters of this equation, activity coefficients for cefazolin sodium in the binary systems were calculated. In ternary solutions, three different DES concentrations (molalities 0.5, 1.0, and 1.5) were used to investigate the effect of concentration on osmotic coefficients. Quantities such as water activity and osmotic coefficients were also obtained for ternary solutions. In the second step, the density and speed of sound data for the binary and ternary solutions were measured at 298.15 K. On the basis of density and speed of sound data, the partial molar volume Vφ and apparent molar isentropic compressibility κφ were calculated. The standard partial molar volume Vφ0 and partial molar isentropic compressibility κφ0 were obtained applying the Redlich–Meyer equation.
中文翻译:

通过蒸气压渗透压法和体积和声学测量了解头孢唑啉钠在氯化胆碱/乙二醇或尿素水溶液中的溶剂化行为
近年来,环保绿色溶剂已成为传统有机溶剂不可缺少的替代品。深共晶溶剂 (DES) 由于其独特的性质在众多用途中受到了极大的关注。这项工作分两步进行。首先,应用蒸气压渗透压测定法测定二元头孢唑啉钠(头孢唑啉钠/水)和三元(头孢唑啉钠/DES:氯化胆碱/乙二醇和氯化胆碱/尿素/水)溶液在T = 298.15 K时的渗透系数和p= 872 hPa。对于二元溶液,测定了水活度、溶液的渗透系数和蒸气压降低。应用Pitzer方程拟合渗透系数数据,根据得到的方程参数,计算出头孢唑啉钠在二元体系中的活度系数。在三元溶液中,使用三种不同的 DES 浓度(摩尔浓度 0.5、1.0 和 1.5)来研究浓度对渗透系数的影响。还获得了三元溶液的水活度和渗透系数等量。第二步,在 298.15 K 处测量二元和三元溶液的声速数据。在声速数据的基础上,得到偏摩尔体积V φ并计算表观摩尔等熵可压缩性 κ φ。应用 Redlich-Meyer 方程获得标准偏摩尔体积V φ 0和偏摩尔等熵可压缩性 κ φ 0 。
更新日期:2022-01-13
中文翻译:

通过蒸气压渗透压法和体积和声学测量了解头孢唑啉钠在氯化胆碱/乙二醇或尿素水溶液中的溶剂化行为
近年来,环保绿色溶剂已成为传统有机溶剂不可缺少的替代品。深共晶溶剂 (DES) 由于其独特的性质在众多用途中受到了极大的关注。这项工作分两步进行。首先,应用蒸气压渗透压测定法测定二元头孢唑啉钠(头孢唑啉钠/水)和三元(头孢唑啉钠/DES:氯化胆碱/乙二醇和氯化胆碱/尿素/水)溶液在T = 298.15 K时的渗透系数和p= 872 hPa。对于二元溶液,测定了水活度、溶液的渗透系数和蒸气压降低。应用Pitzer方程拟合渗透系数数据,根据得到的方程参数,计算出头孢唑啉钠在二元体系中的活度系数。在三元溶液中,使用三种不同的 DES 浓度(摩尔浓度 0.5、1.0 和 1.5)来研究浓度对渗透系数的影响。还获得了三元溶液的水活度和渗透系数等量。第二步,在 298.15 K 处测量二元和三元溶液的声速数据。在声速数据的基础上,得到偏摩尔体积V φ并计算表观摩尔等熵可压缩性 κ φ。应用 Redlich-Meyer 方程获得标准偏摩尔体积V φ 0和偏摩尔等熵可压缩性 κ φ 0 。

























 京公网安备 11010802027423号
京公网安备 11010802027423号