当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective formal carbene insertion into C–N bond of aminal as a concise track to chiral α-amino-β2,2-amino acids and synthetic applications
Green Synthesis and Catalysis Pub Date : 2021-11-1 , DOI: 10.1016/j.gresc.2021.10.007 Xue Tian , Xinfang Xu , Tongfei Jing , Zhenghui Kang , Wenhao Hu
Green Synthesis and Catalysis Pub Date : 2021-11-1 , DOI: 10.1016/j.gresc.2021.10.007 Xue Tian , Xinfang Xu , Tongfei Jing , Zhenghui Kang , Wenhao Hu
|
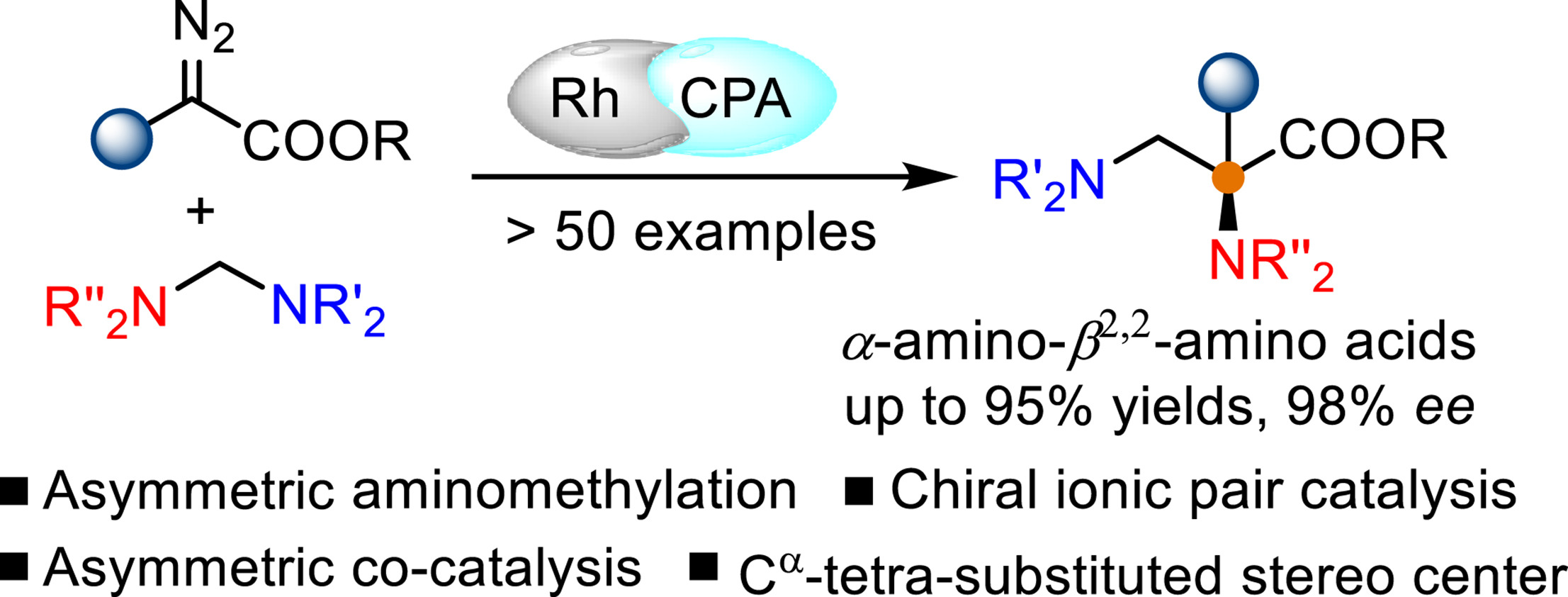
Chiral α-amino-β2,2-amino acid is an ubiquitous fragment that presents in natural products, pharmaceuticals, and bioactive molecules. However, the catalytic enantioselective synthesis of α-amino-β2,2-amino acids with structural complexity and diversity remains a significant challenge. Herein, a highly enantioselective formal C–N bond insertion of aminals with metal carbene species that was generated in situ from diazo compound has been developed by employing a cooperative catalysis of achiral dirhodium complex with chiral phosphonic acid, providing a practical and efficient protocol for expeditious access to optically pure α-amino-β2,2-amino acid derivatives bearing an α-tetra-substituted stereo center with broad substrate scope. The reaction was proposed to proceed through an enantioselective reassembly of enolate intermediate generated in situ from rhodium carbene with methylene iminium ion via chiral ionic pair interaction. Further synthetic applications of these generated poly-functionalized products furnish different types of key intermediates for the formal synthesis of many bioactive molecules. In addition, these amino acid derivatives could also serve as novel potential tail chains of resorcinol dibenzyl either type PD-1/PD-L1 small molecule inhibitors.
中文翻译:

对映选择性形式卡宾插入缩醛胺的 C-N 键作为手性 α-氨基-β2,2-氨基酸和合成应用的简明轨迹
手性 α-氨基-β2,2-氨基酸是一种普遍存在的片段,存在于天然产物、药物和生物活性分子中。然而,具有结构复杂性和多样性的α-氨基-β2,2-氨基酸的催化对映选择性合成仍然是一个重大挑战。在此,通过采用非手性二铑络合物与手性膦酸的协同催化,开发了一种高度对映选择性的形式 C-N 键插入缩醛胺与金属卡宾物质,该化合物是由重氮化合物原位产生的,提供了一种实用且有效的方案,用于快速获得具有广泛底物范围的α-四取代立体中心的光学纯α-氨基-β2,2-氨基酸衍生物。该反应被提议通过手性离子对相互作用通过从铑卡宾与亚甲基亚胺离子原位产生的烯醇化物中间体的对映选择性重组来进行。这些生成的多官能化产品的进一步合成应用为正式合成许多生物活性分子提供了不同类型的关键中间体。此外,这些氨基酸衍生物还可以作为间苯二酚二苄类 PD-1/PD-L1 小分子抑制剂的新型潜在尾链。
更新日期:2022-01-13
中文翻译:

对映选择性形式卡宾插入缩醛胺的 C-N 键作为手性 α-氨基-β2,2-氨基酸和合成应用的简明轨迹
手性 α-氨基-β2,2-氨基酸是一种普遍存在的片段,存在于天然产物、药物和生物活性分子中。然而,具有结构复杂性和多样性的α-氨基-β2,2-氨基酸的催化对映选择性合成仍然是一个重大挑战。在此,通过采用非手性二铑络合物与手性膦酸的协同催化,开发了一种高度对映选择性的形式 C-N 键插入缩醛胺与金属卡宾物质,该化合物是由重氮化合物原位产生的,提供了一种实用且有效的方案,用于快速获得具有广泛底物范围的α-四取代立体中心的光学纯α-氨基-β2,2-氨基酸衍生物。该反应被提议通过手性离子对相互作用通过从铑卡宾与亚甲基亚胺离子原位产生的烯醇化物中间体的对映选择性重组来进行。这些生成的多官能化产品的进一步合成应用为正式合成许多生物活性分子提供了不同类型的关键中间体。此外,这些氨基酸衍生物还可以作为间苯二酚二苄类 PD-1/PD-L1 小分子抑制剂的新型潜在尾链。


























 京公网安备 11010802027423号
京公网安备 11010802027423号