Journal of the Taiwan Institute of Chemical Engineers ( IF 5.7 ) Pub Date : 2021-12-23 , DOI: 10.1016/j.jtice.2021.104179 Yanfang Chen 1 , Xuemei Yan 1 , Haifeng Lin 1 , Chao Wang 1 , Jixiang Xu 1
|
Background
Fe2+/H2O2-based photo-Fenton process is often used in pollutions removal. But, Fe-species related low activity at neutral condition hinders their practical applications. Activation of in situ generated H2O2 for Fenton-like degradation of pollutions through catalysts absences of Fe species is expected.
Methods
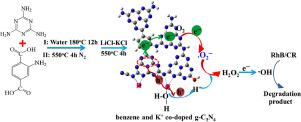
A strip-like benzene and K+ co-doped g-C3N4 (KBCN) was constructed by the thermal polymerization of assembly of melamine and 2-aminoterephthalic acid, then post-calcination in LiCl–KCl molten salt, which was then used to degrade Rhodamine B (RhB) and Congo red (CR) via in situ generated H2O2.
Significant findings
Benzene and K+ co-doping were critical both in increasing charge separation and enhancing the H2O2 production. The yield of H2O2 for KBCN was 57.5 μM in pure water after 60 min irradiation. In the absence of Fe2+, the in situ generated H2O2 could subsequently degrade RhB and CR with the formed ·OH. The apparent rate constants of KBCN for RhB and CR degradation were 0.0446 and 0.0497 min−1, respectively. The advantages of degradation with in situ generated H2O2 in the absence of Fe2+ can provide a new prospective to the environmental friendly Fenton-like reaction.
中文翻译:

苯和 K+ 共掺杂的氮化碳与原位生成的 H2O2 增强类芬顿降解罗丹明 B 和刚果红
背景
Fe 2+ /H 2 O 2基光芬顿法常用于污染物去除。但是,在中性条件下与 Fe 物种相关的低活性阻碍了它们的实际应用。预期通过催化剂不存在 Fe 物种激活原位生成的 H 2 O 2以进行类似芬顿的污染降解。
方法
通过三聚氰胺和2-氨基对苯二甲酸的组装热聚合,然后在LiCl-KCl熔盐中后煅烧,构建了条状苯和K +共掺杂的gC 3 N 4 (KBCN),然后用于通过原位生成的 H 2 O 2降解罗丹明 B (RhB) 和刚果红 (CR) 。
重要发现
苯和K +共掺杂对于增加电荷分离和提高 H 2 O 2产量都至关重要。60 分钟照射后,KBCN的 H 2 O 2在纯水中的产率为 57.5 μM。在不存在的Fe 2+中,在原位生成的ħ 2 ö 2随后可能劣化罗丹明B和CR与形成的·OH。KBCN 对 RhB 和 CR 降解的表观速率常数分别为 0.0446 和 0.0497 min -1。在没有 Fe 的情况下使用原位生成的 H 2 O 2进行降解的优势2+可以为环境友好的类芬顿反应提供新的前景。

























 京公网安备 11010802027423号
京公网安备 11010802027423号