Advanced Drug Delivery Reviews ( IF 16.1 ) Pub Date : 2021-12-20 , DOI: 10.1016/j.addr.2021.114086 Gary L Griffiths 1 , Crystal Vasquez 2 , Freddy Escorcia 2 , Jeff Clanton 3 , Liza Lindenberg 2 , Esther Mena 2 , Peter L Choyke 2
|
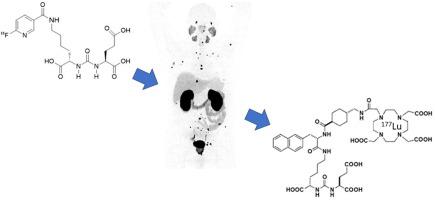
Molecular Imaging is entering the most fruitful, exciting period in its history with many new agents under development, and several reaching the clinic in recent years. While it is unusual for just one laboratory to take an agent from initial discovery through to full clinical approval the steps along the way are important to understand for all interested participants even if one is not involved in the entire process. Here, we provide an overview of these processes beginning at discovery and preclinical validation of a new molecular imaging agent and using as an exemplar a low molecular weight disease-specific targeted positron emission tomography (PET) agent. Compared to standard drug development requirements, molecular imaging agents may benefit from a regulatory standpoint from their low mass administered doses, they nonetheless still need to go through a series of well-defined steps before they can be considered for Phase 1 human testing. After outlining the discovery and preclinical validation approaches, we will also discuss the nuances of Phase 1, Phase 2 and Phase 3 studies that may culminate in an FDA general use approval. Finally, some post-approval aspects of novel molecular imaging agents are considered.
中文翻译:

将放射性标记的显像剂转移到诊所
分子影像学正在进入其历史上最富有成果、最激动人心的时期,许多新药物正在开发中,并且近年来有几种药物进入临床。虽然只有一个实验室从最初的发现到完全临床批准的药物是不寻常的,但对于所有感兴趣的参与者来说,即使没有参与整个过程,了解整个过程中的步骤也很重要。在这里,我们概述了从新分子显像剂的发现和临床前验证开始的这些过程,并以低分子量疾病特异性靶向正电子发射断层扫描 (PET) 试剂为例。与标准药物开发要求相比,分子显像剂可能从监管角度受益于其低质量给药剂量,尽管如此,在考虑进行第一阶段人体测试之前,他们仍然需要经过一系列明确的步骤。在概述了发现和临床前验证方法之后,我们还将讨论可能最终获得 FDA 一般使用批准的 1 期、2 期和 3 期研究的细微差别。最后,考虑了新型分子显像剂的一些批准后方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号