当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iodine-Mediated Fluorination of Alkenes with an HF Reagent: Regioselective Synthesis of 2-Fluoroalkyl Iodides
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02422 Tsugio Kitamura 1 , Ryuichi Komoto 1 , Juzo Oyamada 1 , Masahiro Higashi 2 , Yosuke Kishikawa 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.joc.1c02422 Tsugio Kitamura 1 , Ryuichi Komoto 1 , Juzo Oyamada 1 , Masahiro Higashi 2 , Yosuke Kishikawa 2
Affiliation
|
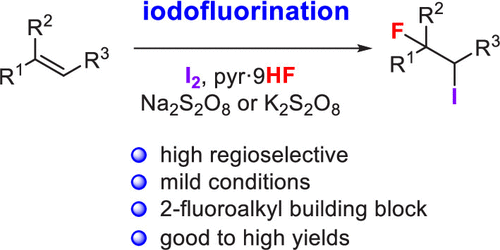
Fluorination reaction of alkenes with iodine and HF·pyridine complex (pyr·9HF) was performed under mild conditions in the presence of K2S2O8 or Na2S2O8 as an oxidant. Aliphatic and aromatic alkenes underwent iodofluorination to give the iodofluorinated products with high regioselectivity. The substitution reactions of the iodofluorinated product by nitrogen, sulfur, and oxygen nucleophiles indicated further applications as a building block for synthesis of 2-fluoroalkyl-substituted compounds.
中文翻译:

用 HF 试剂碘介导的烯烃氟化:2-氟烷基碘化物的区域选择性合成
在K 2 S 2 O 8或Na 2 S 2 O 8氧化剂存在下,烯烃与碘和HF·吡啶络合物(pyr·9HF)的氟化反应在温和条件下进行。脂肪族和芳香族烯烃经过碘氟化反应得到具有高区域选择性的碘氟化产物。氮、硫和氧亲核试剂对碘氟化产物的取代反应表明其进一步用作合成 2-氟烷基取代化合物的构建单元。
更新日期:2021-12-17
中文翻译:

用 HF 试剂碘介导的烯烃氟化:2-氟烷基碘化物的区域选择性合成
在K 2 S 2 O 8或Na 2 S 2 O 8氧化剂存在下,烯烃与碘和HF·吡啶络合物(pyr·9HF)的氟化反应在温和条件下进行。脂肪族和芳香族烯烃经过碘氟化反应得到具有高区域选择性的碘氟化产物。氮、硫和氧亲核试剂对碘氟化产物的取代反应表明其进一步用作合成 2-氟烷基取代化合物的构建单元。



























 京公网安备 11010802027423号
京公网安备 11010802027423号