当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
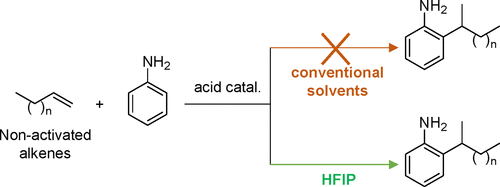
Selective Acid-Catalyzed Hydroarylation of Nonactivated Alkenes with Aniline Assisted by Hexafluoroisopropanol
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-02 , DOI: 10.1021/acs.joc.1c02197 Gongming Peng 1 , Anaelle Humblot 2 , Raphael Wischert 1 , Karine De Oliveira Vigier 2 , Fan Jiang 1 , Marc Pera-Titus 1 , François Jérôme 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-12-02 , DOI: 10.1021/acs.joc.1c02197 Gongming Peng 1 , Anaelle Humblot 2 , Raphael Wischert 1 , Karine De Oliveira Vigier 2 , Fan Jiang 1 , Marc Pera-Titus 1 , François Jérôme 2
Affiliation
|
The catalytic hydroarylation of nonactivated alkenes with aniline is a reaction of high interest, aiming at providing C-functionalized aniline derivatives that are important precursors for the fabrication of polyurethanes. However, this reaction remains a longstanding goal of catalysis, as it requires one to simultaneously address two important goals: (1) the very low reactivity of nonactivated alkenes and (2) control of the hydroarylation/hydroamination selectivity. As a result, the hydroarylation of aniline is mostly restricted to activated alkenes (i.e., featuring ring strain, conjugation, or activation with electron-donating or -withdrawing groups). Here we show that the combination of bismuth triflate and hexafluoroisopropanol (HFIP) leads to the formation of highly active catalytic species capable of promoting the hydroarylation of various nonactivated alkenes, such as 1-octene, 1-heptene, and 1-undecene, among others, with aniline with high selectivity (71–92%). Through a combined experimental and computational investigation, we propose a reaction pathway where HFIP stabilizes the rate-determining transition state through a H-bond interaction with the triflate anion, thus assisting the acid catalyst in the hydroarylation of nonactivated alkenes. From a practical point of view, this work opens a catalytic access to C-functionalized aniline derivatives from two cheap and abundant feedstocks in a 100% atom-economical fashion.
中文翻译:

六氟异丙醇辅助苯胺选择性酸催化未活化烯烃加氢芳基化反应
未活化烯烃与苯胺的催化加氢芳基化反应是一项备受关注的反应,旨在提供 C 官能化的苯胺衍生物,这些衍生物是制造聚氨酯的重要前体。然而,该反应仍然是催化的长期目标,因为它需要同时解决两个重要目标:(1)未活化烯烃的极低反应性和(2)加氢芳基化/加氢胺化选择性的控制。因此,苯胺的加氢芳基化主要限于活化的烯烃(即具有环应变、共轭或用给电子或吸电子基团活化)。在这里,我们展示了三氟甲磺酸铋和六氟异丙醇 (HFIP) 的组合导致形成能够促进各种未活化烯烃(例如 1-辛烯、1-庚烯和 1-十一烯等)加氢芳基化的高活性催化物质, 苯胺具有高选择性 (71–92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。其中,苯胺具有高选择性(71-92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。其中,苯胺具有高选择性(71-92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。从而在未活化烯烃的加氢芳基化中辅助酸催化剂。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。从而在未活化烯烃的加氢芳基化中辅助酸催化剂。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。
更新日期:2021-12-17
中文翻译:

六氟异丙醇辅助苯胺选择性酸催化未活化烯烃加氢芳基化反应
未活化烯烃与苯胺的催化加氢芳基化反应是一项备受关注的反应,旨在提供 C 官能化的苯胺衍生物,这些衍生物是制造聚氨酯的重要前体。然而,该反应仍然是催化的长期目标,因为它需要同时解决两个重要目标:(1)未活化烯烃的极低反应性和(2)加氢芳基化/加氢胺化选择性的控制。因此,苯胺的加氢芳基化主要限于活化的烯烃(即具有环应变、共轭或用给电子或吸电子基团活化)。在这里,我们展示了三氟甲磺酸铋和六氟异丙醇 (HFIP) 的组合导致形成能够促进各种未活化烯烃(例如 1-辛烯、1-庚烯和 1-十一烯等)加氢芳基化的高活性催化物质, 苯胺具有高选择性 (71–92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。其中,苯胺具有高选择性(71-92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。其中,苯胺具有高选择性(71-92%)。通过结合实验和计算研究,我们提出了一种反应途径,其中 HFIP 通过与三氟甲磺酸盐阴离子的 H 键相互作用稳定决定速率的过渡态,从而帮助酸催化剂进行未活化烯烃的加氢芳基化。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。从而在未活化烯烃的加氢芳基化中辅助酸催化剂。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。从而在未活化烯烃的加氢芳基化中辅助酸催化剂。从实用的角度来看,这项工作为从两种廉价且丰富的原料中以 100% 原子经济的方式催化获得 C 官能化苯胺衍生物开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号