Water Research ( IF 12.8 ) Pub Date : 2021-12-03 , DOI: 10.1016/j.watres.2021.117931 Jens Terhalle 1 , Simon E Nikutta 1 , Dawid L Krzeciesa 1 , Holger V Lutze 2 , Maik A Jochmann 3 , Torsten C Schmidt 4
|
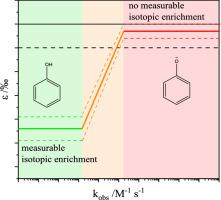
Ozonation is nowadays a widely used method in drinking water treatment for disinfection and pollutant control. However, transformation products of ozonation can be more toxic than their parent compounds. Therefore, the knowledge of the reaction mechanisms and product formation is essential for a safe application. Different analytical methods such as high-resolution mass spectrometry (HRMS) and compound-specific isotope analysis (CSIA) can be applied to elucidate products and primary attack positions of oxidation agents such as ozone. During the investigation of the ozonation of phenolic compounds in water by CSIA, a reaction rate depending carbon isotope fractionation was observed. The fractionation strongly depends on the phenol speciation. With decreasing pH values and reaction rates <105 M−1 s−1, the isotope enrichment factor ε increases (ε is between -5.2 and -1.0‰). For faster reactions (>105 M−1 s−1), the carbon isotope enrichment was not significant anymore (ε is between -1.0 and 0‰). Based on these data a concept to correlate isotope enrichment factors with kinetic data for aromatic compounds is proposed. The additional investigation of aliphatic double and triple bonds did not fit this correlation suggesting different rate-limiting steps. However, double and triple bond showed a similar enrichment factor, which implies the same rate-limiting step in the reaction with ozone, the monodentate addition of ozone.
中文翻译:

使用酚作为探针将臭氧化反应的反应速率常数和同位素分馏联系起来
臭氧化是当今饮用水处理中广泛使用的消毒和污染物控制方法。然而,臭氧化的转化产物可能比它们的母体化合物毒性更大。因此,了解反应机理和产物形成对于安全应用至关重要。高分辨率质谱 (HRMS) 和化合物特异性同位素分析 (CSIA) 等不同的分析方法可用于阐明臭氧等氧化剂的产物和主要攻击位置。在通过 CSIA 研究水中酚类化合物的臭氧化过程中,观察到反应速率取决于碳同位素分馏。分馏很大程度上取决于苯酚的形态。随着 pH 值降低和反应速率 <10 5 M -1s -1,同位素富集因子ε增加(ε在-5.2和-1.0‰之间)。对于更快的反应(>10 5 M -1 s -1),碳同位素富集不再显着(ε 介于 -1.0 和 0‰ 之间)。基于这些数据,提出了将同位素富集因子与芳族化合物的动力学数据相关联的概念。脂肪族双键和三键的额外研究不符合这种相关性,表明不同的限速步骤。然而,双键和三键显示出相似的富集因子,这意味着与臭氧反应的限速步骤相同,即臭氧的单齿加成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号