当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineered Nanoconfinement Accelerating Spontaneous Manganese-Catalyzed Degradation of Organic Contaminants
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-12-01 , DOI: 10.1021/acs.est.1c06551 Shuo Zhang 1 , Tayler Hedtke 1 , Li Wang 1 , Xiaoxiong Wang 1 , Tianchi Cao 1 , Menachem Elimelech 1 , Jae-Hong Kim 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-12-01 , DOI: 10.1021/acs.est.1c06551 Shuo Zhang 1 , Tayler Hedtke 1 , Li Wang 1 , Xiaoxiong Wang 1 , Tianchi Cao 1 , Menachem Elimelech 1 , Jae-Hong Kim 1
Affiliation
|
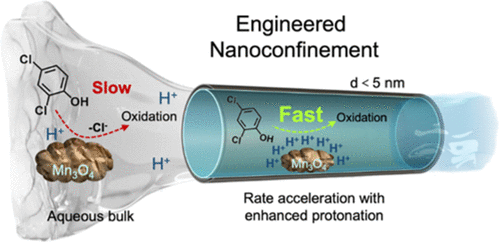
Manganese(III/IV) oxide minerals are known to spontaneously degrade organic pollutants in nature. However, the kinetics are too slow to be useful for engineered water treatment processes. Herein, we demonstrate that nanoscale Mn3O4 particles under nanoscale spatial confinement (down to 3–5 nm) can significantly accelerate the kinetics of pollutant degradation, nearly 3 orders of magnitude faster compared to the same reaction in the unconfined bulk phase. We first employed an anodized aluminum oxide scaffold with uniform channel dimensions for experimental and computational studies. We found that the observed kinetic enhancement resulted from the increased surface area of catalysts exposed to the reaction, as well as the increased local proton concentration at the Mn3O4 surface and subsequent acceleration of acid-catalyzed reactions even at neutral pH in bulk. We further demonstrate that a reactive Mn3O4-functionalized ceramic ultrafiltration membrane, a more suitable scaffold for realistic water treatment, achieved nearly complete removal of various phenolic and aniline pollutants, operated under a common ultrafiltration water flux. Our findings mark an important advance toward the development of catalytic membranes that can degrade pollutants in addition to their intrinsic function as a physical separation barrier, especially since they are based on accelerating natural catalytic pathways that do not require any chemical addition.
中文翻译:

工程纳米限制加速有机污染物的自发性锰催化降解
已知氧化锰 (III/IV) 矿物会自发降解自然界中的有机污染物。然而,动力学太慢而不能用于工程水处理过程。在这里,我们证明了在纳米级空间限制下(低至 3-5 nm)的纳米级 Mn 3 O 4颗粒可以显着加速污染物降解的动力学,与无限制体相中的相同反应相比快了近 3 个数量级。我们首先使用具有均匀通道尺寸的阳极氧化铝支架进行实验和计算研究。我们发现观察到的动力学增强是由于暴露于反应的催化剂表面积增加,以及 Mn 3处局部质子浓度增加所致。即使在中性 pH 值下,O 4表面和随后的酸催化反应加速。我们进一步证明了反应性 Mn 3 O 4功能化陶瓷超滤膜(一种更适合实际水处理的支架)在普通超滤水通量下几乎完全去除了各种酚类和苯胺类污染物。我们的研究结果标志着催化膜的开发取得了重要进展,该膜除了作为物理分离屏障的内在功能外,还可以降解污染物,特别是因为它们基于不需要任何化学添加的加速天然催化途径。
更新日期:2021-12-21
中文翻译:

工程纳米限制加速有机污染物的自发性锰催化降解
已知氧化锰 (III/IV) 矿物会自发降解自然界中的有机污染物。然而,动力学太慢而不能用于工程水处理过程。在这里,我们证明了在纳米级空间限制下(低至 3-5 nm)的纳米级 Mn 3 O 4颗粒可以显着加速污染物降解的动力学,与无限制体相中的相同反应相比快了近 3 个数量级。我们首先使用具有均匀通道尺寸的阳极氧化铝支架进行实验和计算研究。我们发现观察到的动力学增强是由于暴露于反应的催化剂表面积增加,以及 Mn 3处局部质子浓度增加所致。即使在中性 pH 值下,O 4表面和随后的酸催化反应加速。我们进一步证明了反应性 Mn 3 O 4功能化陶瓷超滤膜(一种更适合实际水处理的支架)在普通超滤水通量下几乎完全去除了各种酚类和苯胺类污染物。我们的研究结果标志着催化膜的开发取得了重要进展,该膜除了作为物理分离屏障的内在功能外,还可以降解污染物,特别是因为它们基于不需要任何化学添加的加速天然催化途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号