European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2021-12-01 , DOI: 10.1016/j.ejmech.2021.114031 Bo Ren 1 , Cong Guo 1 , Run-Ze Liu 1 , Zhao-Yuan Bian 1 , Rong-Chun Liu 1 , Lan-Fang Huang 1 , Jiang-Jiang Tang 1
|
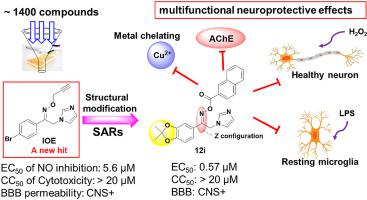
Alzheimer's disease (AD) possesses a complex pathogenetic mechanism. Nowadays, multitarget agents are considered to have potential in effectively treating AD via triggering molecules in functionally complementary pathways at the same time. Here, based on the screening (∼1400 compounds) against neuroinflammation, an imidazolylacetophenone oxime ether (IOE) was discovered as a novel hit. In order to obtain SARs, a series of imidazolylacetophenone oxime derivatives were constructed, and their C=N bonds were confirmed as the Z configuration by single crystals. These derivatives exhibited potential multifunctional neuroprotective effects including anti-neuroinflammatory, antioxidative damage, metal-chelating, inhibition of acetylcholinesterase (AChE) properties. Among these derivatives, compound 12i displayed the most potent inhibitory activity against nitric oxide (NO) production with EC50 value of 0.57 μM 12i can dose-dependently suppress the expression of iNOS and COX-2 but not change the expression of HO-1 protein. Moreover, 12i exhibited evidently neuroprotective effects on H2O2-induced PC12 cells damage and ferroptosis without cytotoxicity at 10 μM, as well as selectively metal chelating properties via chelating Cu2+. In addition, 12i showed a mixed-type inhibitory effect on AChE in vitro. The structure-activity relationships (SARs) analysis indicated that dioxolane groups on benzene ring and rigid oxime ester can improve the activity. Parallel artificial membrane permeation assay (PAMPA) also verified that 12i can overcome the blood-brain barrier (BBB). Overall, this is the first report on imidazolylacetophenone oxime-based multifunctional neuroprotective effects, suggesting that this type of compounds might be novel multifunctional agents against AD.
中文翻译:

基于咪唑基苯乙酮肟的多功能神经保护剂:发现和构效关系
阿尔茨海默病(AD)具有复杂的发病机制。如今,多靶点药物被认为具有通过同时触发功能互补途径中的分子来有效治疗 AD 的潜力。在这里,基于针对神经炎症的筛选(约 1400 种化合物),发现了一种新型的咪唑基苯乙酮肟醚 ( IOE )。为了获得SARs,构建了一系列咪唑基苯乙酮肟衍生物,并确认它们的C=N键为Z通过单晶配置。这些衍生物表现出潜在的多功能神经保护作用,包括抗神经炎症、抗氧化损伤、金属螯合、抑制乙酰胆碱酯酶 (AChE) 特性。在这些衍生物中,化合物12i对一氧化氮 (NO) 产生最有效的抑制活性,EC 50值为 0.57 μM 12i可以剂量依赖性地抑制 iNOS 和 COX-2 的表达,但不改变 HO-1 蛋白的表达. 此外,12i对H 2 O 2表现出明显的神经保护作用- 诱导的 PC12 细胞损伤和铁死亡,在 10 μM 时没有细胞毒性,以及通过螯合 Cu 2+的选择性金属螯合特性。此外,12i在体外对 AChE 显示出混合型抑制作用。构效关系(SARs)分析表明苯环上的二氧戊环和刚性肟酯可以提高活性。平行人工膜渗透试验 (PAMPA) 也验证了12i可以克服血脑屏障 (BBB)。总体而言,这是关于基于咪唑基苯乙酮肟的多功能神经保护作用的第一份报告,表明此类化合物可能是针对 AD 的新型多功能药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号