当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
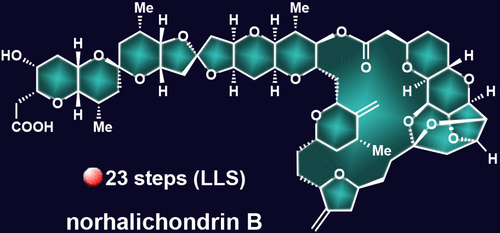
A Highly Convergent Total Synthesis of Norhalichondrin B
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-12-01 , DOI: 10.1021/jacs.1c10539 K C Nicolaou 1 , Saiyong Pan 1 , Yogesh Shelke 1 , Qiuji Ye 1 , Dipendu Das 1 , Stephan Rigol 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-12-01 , DOI: 10.1021/jacs.1c10539 K C Nicolaou 1 , Saiyong Pan 1 , Yogesh Shelke 1 , Qiuji Ye 1 , Dipendu Das 1 , Stephan Rigol 1
Affiliation
|
A new synthetic strategy for the total synthesis of norhalichondrin B featuring a highly convergent approach and our recently disclosed reverse approach for the synthesis of cyclic ether structural motifs is disclosed. Resulting in the shortest route to norhalichondrin B disclosed thus far, the reported total synthesis was achieved through the synthesis of two almost equally complex fragments whose coupling and short elaboration sequence featured an essential epimerization of the C16 stereocenter occurring concurrently with a simple acid-induced deprotection, a tactic based on a prior study along the synthetic route. This unprecedented strategy within the halichondrin family of natural products could find practical application to the synthesis of other more or less complex natural or designed halichondrin analogues.
中文翻译:

去甲软骨素 B 的高度收敛全合成
公开了一种用于全合成去甲软骨素 B 的新合成策略,该策略具有高度收敛的方法和我们最近公开的用于合成环醚结构基序的反向方法。导致迄今为止公开的去甲软硬脂素 B 的最短途径,所报道的全合成是通过合成两个几乎同样复杂的片段来实现的,这些片段的偶联和短细化序列的特点是 C16 立体中心的基本差向异构化与简单的酸诱导的脱保护同时发生,一种基于合成路线先前研究的策略。这种在软软骨素家族天然产物中前所未有的策略可以在其他或多或少复杂的天然或设计的软软骨素类似物的合成中找到实际应用。
更新日期:2021-12-15
中文翻译:

去甲软骨素 B 的高度收敛全合成
公开了一种用于全合成去甲软骨素 B 的新合成策略,该策略具有高度收敛的方法和我们最近公开的用于合成环醚结构基序的反向方法。导致迄今为止公开的去甲软硬脂素 B 的最短途径,所报道的全合成是通过合成两个几乎同样复杂的片段来实现的,这些片段的偶联和短细化序列的特点是 C16 立体中心的基本差向异构化与简单的酸诱导的脱保护同时发生,一种基于合成路线先前研究的策略。这种在软软骨素家族天然产物中前所未有的策略可以在其他或多或少复杂的天然或设计的软软骨素类似物的合成中找到实际应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号