当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The influence of the cation structure on the basicity-related polarity of ionic liquids
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1cp03986e Nadine Weiß 1 , Gabi Thielemann 1 , Kevin Nagel 1 , Caroline H Schmidt 1 , Andreas Seifert 1 , Lysann Kaßner 1 , Veronika Strehmel 2 , Björn Corzilius 3 , Christian Schröder 4 , Stefan Spange 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1cp03986e Nadine Weiß 1 , Gabi Thielemann 1 , Kevin Nagel 1 , Caroline H Schmidt 1 , Andreas Seifert 1 , Lysann Kaßner 1 , Veronika Strehmel 2 , Björn Corzilius 3 , Christian Schröder 4 , Stefan Spange 1
Affiliation
|
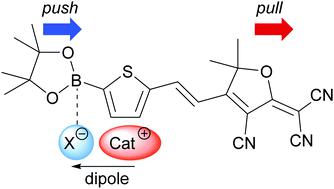
UV/Vis absorption data of (E)-4-(2-[5-{4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl}thiene-2-yl]vinyl)-2-(dicyano-methylene)-3-cyano-5,5-dimethyl-2,5-dihydrofuran (ThTCF) as a solvatochromic probe is applied to examine the anion coordination strength (e.g. of N(CN)2, BF4, PF6, N(Tf)2, CF3COO) as a function of the cation structure of ionic liquids. Several 1-n-alky-3-methylimidazolium- and tetraalkylammonium CH3-NR3+-based ILs with different n-alkyl chain lengths (R = –C4H9, –C6H11, –C8H17, –C10H21) are considered. UV/Vis absorption data of ThTCF show subtle correlations with hydrogen bond accepting (HBA) ability-related measurands such as Kamlet–Taft β, Freire's EHB, and Laurence β1 parameter as a function of anion and cation structure. The different influence of the n-alkyl chain length of imidazolium- and tetraalkylammonium-based ILs on the dipolarity and HBA strength is confirmed by comparison with the 14N isotropic hyperfine coupling constants (Aiso) of a positively (CATI) and negatively charged spin probe (TSKCr) of TEMPO-type [(2,2,6,6-tetramethylpiperidin-1-yl)oxyl] and quantum chemically derived dipoles of the cations. The Aiso values correlate with the absorption energy of ThTCF and EHB, but in different ways depending on the anion or charge of the spin probe. In a final discussion of the β, EHB, and β1 scales in relation to ThTCF, the importance of the molar concentration N of ionic liquids for the physical significance of the respective parameters is discussed.
中文翻译:

阳离子结构对离子液体碱性极性的影响
( E )-4-(2-[5-{4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl}thiene-2-yl]vinyl)-的UV/Vis吸收数据2-(二氰基-亚甲基)-3-氰基-5,5-二甲基-2,5-二氢呋喃 ( ThTCF ) 作为溶剂致变色探针用于检测阴离子配位强度 (例如N(CN) 2 , BF 4 , PF 6、N(Tf) 2、CF 3 COO) 作为离子液体的阳离子结构的函数。几个1- Ñ -alky -3- methylimidazolium-和四烷基铵CH 3 -NR 3 +基离子液体具有不同ñ -烷基链长度(R = -C 4 H ^ 9, –C 6 H 11 , –C 8 H 17 , –C 10 H 21 ) 被考虑。ThTCF 的UV/Vis 吸收数据显示与氢键接受 (HBA) 能力相关的测量值(如 Kamlet-Taft β、Freire 的E HB和 Laurence β 1参数作为阴离子和阳离子结构的函数)之间存在微妙的相关性。所述的不同的影响Ñ imidazolium-的烷基链长和基于四烷基铵,离子液体在偶极性和HBA强度通过与比较确认14个Ñ各向同性超精细耦合常数(甲iso ) 正 ( CATI ) 和带负电的 TEMPO 型 [(2,2,6,6-四甲基哌啶-1-基)氧基] 的自旋探针 ( TSKCr ) 和阳离子的量子化学衍生偶极子。的甲异值与吸收能量相关ThTCF和ë HB,但以不同的方式取决于自旋探头的阴离子或电荷。在与ThTCF相关的β、 E HB和β 1尺度的最后讨论中,摩尔浓度N的重要性 离子液体对于各个参数的物理意义进行了讨论。
更新日期:2021-11-30
中文翻译:

阳离子结构对离子液体碱性极性的影响
( E )-4-(2-[5-{4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl}thiene-2-yl]vinyl)-的UV/Vis吸收数据2-(二氰基-亚甲基)-3-氰基-5,5-二甲基-2,5-二氢呋喃 ( ThTCF ) 作为溶剂致变色探针用于检测阴离子配位强度 (例如N(CN) 2 , BF 4 , PF 6、N(Tf) 2、CF 3 COO) 作为离子液体的阳离子结构的函数。几个1- Ñ -alky -3- methylimidazolium-和四烷基铵CH 3 -NR 3 +基离子液体具有不同ñ -烷基链长度(R = -C 4 H ^ 9, –C 6 H 11 , –C 8 H 17 , –C 10 H 21 ) 被考虑。ThTCF 的UV/Vis 吸收数据显示与氢键接受 (HBA) 能力相关的测量值(如 Kamlet-Taft β、Freire 的E HB和 Laurence β 1参数作为阴离子和阳离子结构的函数)之间存在微妙的相关性。所述的不同的影响Ñ imidazolium-的烷基链长和基于四烷基铵,离子液体在偶极性和HBA强度通过与比较确认14个Ñ各向同性超精细耦合常数(甲iso ) 正 ( CATI ) 和带负电的 TEMPO 型 [(2,2,6,6-四甲基哌啶-1-基)氧基] 的自旋探针 ( TSKCr ) 和阳离子的量子化学衍生偶极子。的甲异值与吸收能量相关ThTCF和ë HB,但以不同的方式取决于自旋探头的阴离子或电荷。在与ThTCF相关的β、 E HB和β 1尺度的最后讨论中,摩尔浓度N的重要性 离子液体对于各个参数的物理意义进行了讨论。


























 京公网安备 11010802027423号
京公网安备 11010802027423号