当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroelectrochemical study of the reduction of 2-methyl-9H-thioxanthene-9-one and its S,S-dioxide and electronic absorption spectra of their molecular ions
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-15 , DOI: 10.1039/d1cp04464h Danila S Odintsov 1 , Inna K Shundrina 1 , Dmitry E Gorbunov 2 , Nina P Gritsan 2 , Jens Beckmann 3 , Leonid A Shundrin 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-15 , DOI: 10.1039/d1cp04464h Danila S Odintsov 1 , Inna K Shundrina 1 , Dmitry E Gorbunov 2 , Nina P Gritsan 2 , Jens Beckmann 3 , Leonid A Shundrin 1
Affiliation
|
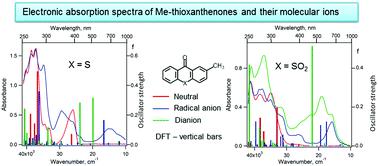
2-Methyl-9H-thioxanthene-9-one (1) and its S,S-dioxide (2) are the precursors of pendant groups that determine the reduction potentials of electro-active polyimides, which exhibit electrochromic behavior and are used in organic electronics. Electrochemical reduction of 1 and 2 leads to the formation of the corresponding persistent radical anions and dianion (for S,S-dioxide). Using 3D spectroelectrochemistry, all anions have been shown to exhibit strong absorption in the UV-VIS-NIR wavelength region. Electronic absorption spectra of 1 and 2 and their negative ions were interpreted using time-dependent DFT. According to the calculations, the most intense electronic transitions of the dianions 12− and 22− in the visible region exhibit hypsochromic shift compared to the intense transitions of the corresponding radical anions and have much higher oscillator strengths, which was confirmed experimentally for 2. An empirical kinetic model was proposed based on the analysis of the total charge passed through the cell during electrolysis and on the established mechanism of electrochemical reduction. This model perfectly described the UV-VIS-NIR optical density time dependences observed on 3D spectroelectrochemical surfaces for both compounds 1 and 2. This made it possible to explain the differences in the electrochromic behaviour of ambibolar electro-active polyimides with pendant groups based on 1, 2.
中文翻译:

2-甲基-9H-噻吨-9-one还原及其S,S-二氧化物的光谱电化学研究及其分子离子的电子吸收光谱
2-Methyl-9 H -thioxanthene-9-one ( 1 ) 及其 S,S-dioxide ( 2 ) 是决定电活性聚酰亚胺还原电位的侧基的前体,其表现出电致变色行为并用于有机电子产品。1和2 的电化学还原导致形成相应的持久性自由基阴离子和二价阴离子(对于S,S - 二氧化物)。使用 3D 光谱电化学,所有阴离子都显示出在 UV-VIS-NIR 波长范围内的强吸收。1和2的电子吸收光谱并使用时间相关的 DFT 解释它们的负离子。根据计算,可见光区二价阴离子1 2-和2 2-最强烈的电子跃迁与相应自由基阴离子的强烈跃迁相比表现出低色移,并且具有更高的振子强度,这在实验中得到了2 . 基于对电解过程中通过电池的总电荷的分析和已建立的电化学还原机制,提出了一种经验动力学模型。该模型完美地描述了在 3D 光谱电化学表面上观察到的化合物1和2 . 这使得可以解释具有基于1、2 的侧基的双分子电活性聚酰亚胺的电致变色行为的差异。
更新日期:2021-11-30
中文翻译:

2-甲基-9H-噻吨-9-one还原及其S,S-二氧化物的光谱电化学研究及其分子离子的电子吸收光谱
2-Methyl-9 H -thioxanthene-9-one ( 1 ) 及其 S,S-dioxide ( 2 ) 是决定电活性聚酰亚胺还原电位的侧基的前体,其表现出电致变色行为并用于有机电子产品。1和2 的电化学还原导致形成相应的持久性自由基阴离子和二价阴离子(对于S,S - 二氧化物)。使用 3D 光谱电化学,所有阴离子都显示出在 UV-VIS-NIR 波长范围内的强吸收。1和2的电子吸收光谱并使用时间相关的 DFT 解释它们的负离子。根据计算,可见光区二价阴离子1 2-和2 2-最强烈的电子跃迁与相应自由基阴离子的强烈跃迁相比表现出低色移,并且具有更高的振子强度,这在实验中得到了2 . 基于对电解过程中通过电池的总电荷的分析和已建立的电化学还原机制,提出了一种经验动力学模型。该模型完美地描述了在 3D 光谱电化学表面上观察到的化合物1和2 . 这使得可以解释具有基于1、2 的侧基的双分子电活性聚酰亚胺的电致变色行为的差异。


























 京公网安备 11010802027423号
京公网安备 11010802027423号