当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Remarkably high solvatochromism in the circular dichroism spectra of the polyproline-II conformation: limitations or new opportunities?
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-22 , DOI: 10.1039/d1cp04551b Vladimir Kubyshkin 1 , Jochen Bürck 2 , Oleg Babii 2 , Nediljko Budisa 1, 3 , Anne S Ulrich 2, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-22 , DOI: 10.1039/d1cp04551b Vladimir Kubyshkin 1 , Jochen Bürck 2 , Oleg Babii 2 , Nediljko Budisa 1, 3 , Anne S Ulrich 2, 4
Affiliation
|
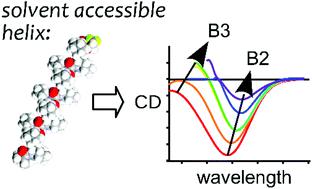
Circular dichroism is a conventional method for studying the secondary structures of peptides and proteins and their transitions. While certain circular dichroism features are characteristic of α-helices and β-strands, the third most abundant secondary structure, the polyproline-II helix, does not exhibit a strictly conserved spectroscopic appearance. Due to its extended nature, the polyproline-II helix is highly accessible to the surrounding solvent; thus, the environment has a critical influence on the lineshape of the circular dichroism spectra of this structure. To showcase possible effects due to the medium, in this work, we report an experimental spectroscopic study of polyproline-II-forming oligomeric peptides in various environments: solvents, detergent micelles, and liposomes. Strikingly, the examination of an oligomeric peptide in a solvent series showed a remarkable 7 nm solvatochromic shift in the main negative band starting with hexafluoropropan-2-ol and moving to hexane. Furthermore, a previously predicted positive band below 200 nm was discovered in the spectra in nonpolar environments. In isotropic liposomes, the expected transition to the transmembrane state correlated with the appearance of a positive band at 228 nm. Our results demonstrate that changes in solvation should be taken into consideration when assessing the circular dichroism spectra of peptides expected to adopt the polyproline-II conformation. Although this precaution may complicate spectral analysis, characterization of solvent-induced spectral changes can generate new opportunities for testing the location of peptides in complex systems such as micelles or lipid bilayers.
中文翻译:

聚脯氨酸-II构象的圆二色光谱中显着的高溶剂致变色性:限制还是新机遇?
圆二色性是研究肽和蛋白质的二级结构及其跃迁的常规方法。虽然某些圆二色性特征是 α-螺旋和 β-链的特征,但第三丰富的二级结构 polyproline-II 螺旋并未表现出严格保守的光谱外观。由于其扩展性质,聚脯氨酸-II 螺旋很容易被周围的溶剂所接触;因此,环境对该结构的圆二色光谱的线形有重要影响。为了展示介质可能产生的影响,在这项工作中,我们报告了在各种环境中形成聚脯氨酸-II 的寡聚肽的实验光谱研究:溶剂、洗涤剂胶束和脂质体。引人注目的是,对溶剂系列中的寡聚肽的检查表明,从 hexafluoropropan-2-ol 开始并移动到己烷的主要负带中存在显着的 7 nm 溶剂化变色位移。此外,在非极性环境中的光谱中发现了先前预测的低于 200 nm 的正波段。在各向同性脂质体中,向跨膜状态的预期转变与 228 nm 处阳性带的出现相关。我们的结果表明,在评估预期采用多脯氨酸-II 构象的肽的圆二色光谱时,应考虑溶剂化的变化。尽管这种预防措施可能会使光谱分析复杂化,
更新日期:2021-11-26
中文翻译:

聚脯氨酸-II构象的圆二色光谱中显着的高溶剂致变色性:限制还是新机遇?
圆二色性是研究肽和蛋白质的二级结构及其跃迁的常规方法。虽然某些圆二色性特征是 α-螺旋和 β-链的特征,但第三丰富的二级结构 polyproline-II 螺旋并未表现出严格保守的光谱外观。由于其扩展性质,聚脯氨酸-II 螺旋很容易被周围的溶剂所接触;因此,环境对该结构的圆二色光谱的线形有重要影响。为了展示介质可能产生的影响,在这项工作中,我们报告了在各种环境中形成聚脯氨酸-II 的寡聚肽的实验光谱研究:溶剂、洗涤剂胶束和脂质体。引人注目的是,对溶剂系列中的寡聚肽的检查表明,从 hexafluoropropan-2-ol 开始并移动到己烷的主要负带中存在显着的 7 nm 溶剂化变色位移。此外,在非极性环境中的光谱中发现了先前预测的低于 200 nm 的正波段。在各向同性脂质体中,向跨膜状态的预期转变与 228 nm 处阳性带的出现相关。我们的结果表明,在评估预期采用多脯氨酸-II 构象的肽的圆二色光谱时,应考虑溶剂化的变化。尽管这种预防措施可能会使光谱分析复杂化,


























 京公网安备 11010802027423号
京公网安备 11010802027423号