当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd(II)-Catalyzed Synthesis of Benzocyclobutenes by β-Methylene-Selective C(sp3)–H Arylation with a Transient Directing Group
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-24 , DOI: 10.1021/jacs.1c09368 Philip A Provencher 1 , John F Hoskin 1 , Jonathan J Wong 2 , Xiangyang Chen 2 , Jin-Quan Yu 3 , K N Houk 2 , Erik J Sorensen 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-24 , DOI: 10.1021/jacs.1c09368 Philip A Provencher 1 , John F Hoskin 1 , Jonathan J Wong 2 , Xiangyang Chen 2 , Jin-Quan Yu 3 , K N Houk 2 , Erik J Sorensen 1
Affiliation
|
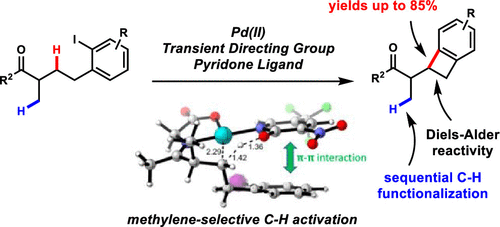
Methylene-selective C–H functionalization is a significant hurdle that remains to be addressed in the field of Pd(II) catalysis. We report a Pd(II)-catalyzed synthesis of benzocyclobutenes by methylene-selective C(sp3)–H arylation of ketones. The reaction utilizes glycine as a transient directing group and a 2-pyridone ligand, which may govern the methylene selectivity by making intimate molecular associations with the substrate during concerted metalation–deprotonation. This reaction is shown to be highly selective for intramolecular methylene C(sp3)–H arylation, thus enabling sequential C(sp3)–H functionalization.
中文翻译:

Pd(II)-催化β-亚甲基选择性C(sp3)-H芳基化与瞬态导向基团合成苯并环丁烯
亚甲基选择性 C-H 官能化是 Pd(II) 催化领域仍有待解决的重大障碍。我们报告了通过酮的亚甲基选择性 C(sp 3 )-H 芳基化合成苯并环丁烯的 Pd(II) 催化。该反应利用甘氨酸作为瞬态导向基团和 2-吡啶酮配体,这可以通过在协同金属化-去质子化过程中与底物形成紧密的分子结合来控制亚甲基的选择性。该反应被证明对分子内亚甲基 C(sp 3 )-H 芳基化具有高度选择性,因此能够实现连续的 C(sp 3 )-H 官能化。
更新日期:2021-12-08
中文翻译:

Pd(II)-催化β-亚甲基选择性C(sp3)-H芳基化与瞬态导向基团合成苯并环丁烯
亚甲基选择性 C-H 官能化是 Pd(II) 催化领域仍有待解决的重大障碍。我们报告了通过酮的亚甲基选择性 C(sp 3 )-H 芳基化合成苯并环丁烯的 Pd(II) 催化。该反应利用甘氨酸作为瞬态导向基团和 2-吡啶酮配体,这可以通过在协同金属化-去质子化过程中与底物形成紧密的分子结合来控制亚甲基的选择性。该反应被证明对分子内亚甲基 C(sp 3 )-H 芳基化具有高度选择性,因此能够实现连续的 C(sp 3 )-H 官能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号