当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral, sequence-definable foldamer-derived macrocycles
Chemical Science ( IF 8.4 ) Pub Date : 2021-11-10 , DOI: 10.1039/d1sc05021d Toyah M C Warnock 1 , Sundaram Rajkumar 2 , Matthew P Fitzpatrick 1 , Christopher J Serpell 3 , Paul Dingwall 1 , Peter C Knipe 1
Chemical Science ( IF 8.4 ) Pub Date : 2021-11-10 , DOI: 10.1039/d1sc05021d Toyah M C Warnock 1 , Sundaram Rajkumar 2 , Matthew P Fitzpatrick 1 , Christopher J Serpell 3 , Paul Dingwall 1 , Peter C Knipe 1
Affiliation
|
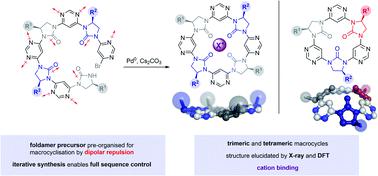
Nature's oligomeric macromolecules have been a long-standing source of inspiration for chemists producing foldamers. Natural systems are frequently conformationally stabilised by macrocyclisation, yet this approach has been rarely adopted in the field of foldamer chemistry. Here we present a new class of chiral cyclic trimers and tetramers formed by macrocyclisation of open-chain foldamer precursors. Symmetrical products are obtained via a [2 + 2] self-assembly approach, while full sequence control is demonstrated through linear synthesis and cyclisation of an unsymmetrical trimer. Structural characterisation is achieved through a combined X-ray and DFT approach, which indicates the tetramers adopt a near-planar conformation, while the trimers adopt a shallow bowl-like shape. Finally, a proof-of-concept experiment is conducted to demonstrate the macrocycles' capacity for cation binding.
中文翻译:

手性、可序列定义的折叠体衍生的大环化合物
大自然的低聚大分子长期以来一直是生产折叠分子的化学家的灵感来源。自然系统经常通过大环化来稳定构象,但这种方法在折叠分子化学领域很少采用。在这里,我们提出了一类新的手性环状三聚体和四聚体,由开链折叠前体的大环化形成。对称的产品获得通过一种[2 + 2]自组装方法,而通过不对称三聚体的线性合成和环化证明了完整的序列控制。结构表征是通过组合 X 射线和 DFT 方法实现的,这表明四聚体采用近平面构象,而三聚体采用浅碗状形状。最后,进行概念验证实验以证明大环化合物的阳离子结合能力。
更新日期:2021-11-25
中文翻译:

手性、可序列定义的折叠体衍生的大环化合物
大自然的低聚大分子长期以来一直是生产折叠分子的化学家的灵感来源。自然系统经常通过大环化来稳定构象,但这种方法在折叠分子化学领域很少采用。在这里,我们提出了一类新的手性环状三聚体和四聚体,由开链折叠前体的大环化形成。对称的产品获得通过一种[2 + 2]自组装方法,而通过不对称三聚体的线性合成和环化证明了完整的序列控制。结构表征是通过组合 X 射线和 DFT 方法实现的,这表明四聚体采用近平面构象,而三聚体采用浅碗状形状。最后,进行概念验证实验以证明大环化合物的阳离子结合能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号