Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2021-11-24 , DOI: 10.1016/j.jpha.2021.11.005 Min Pei 1, 2 , Tingting Liu 3 , Lu Ouyang 3 , Jianhua Sun 3 , Xiaojie Deng 3 , Xiaomin Sun 3 , Wei Wu 3 , Peng Huang 1 , Yi-Li Chen 4 , Xiaorong Tan 1 , Xiaoyue Liu 1, 2 , Peng Zhu 3 , Yongzhen Liu 3 , Deheng Wang 3 , Junliang Wu 3 , Qi Wang 1 , Guifeng Wang 1 , Likun Gong 2, 3, 5 , Qiuping Qin 3 , Chunhe Wang 1, 2, 4, 6
|
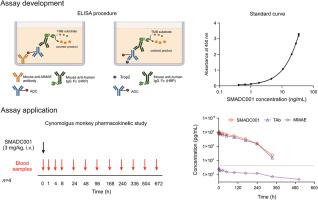
Antibody-drug conjugates (ADCs) are commonly heterogeneous and require extensive assessment of exposure-efficacy and exposure-safety relationships in preclinical and clinical studies. In this study, we report the generation of a monoclonal antibody against monomethyl auristatin E (MMAE) and the development, validation, and application of sensitive and high-throughput enzyme-linked immunosorbent assays (ELISA) to measure the concentrations of MMAE-conjugated ADCs and total antibodies (tAb, antibodies in ADC plus unconjugated antibodies) in cynomolgus monkey sera. These assays were successfully applied to in vitro plasma stability and pharmacokinetic (PK) studies of SMADC001, an MMAE-conjugated ADC against trophoblast cell surface antigen 2 (TROP-2). The plasma stability of SMADC001 was better than that of similar ADCs coupled with PEG4-Val-Cit, Lys (m-dPEG24)-Cit, and Val-Cit linkers. The developed ELISA methods for the calibration standards of ADC and tAb revealed a correlation between serum concentrations and the OD450 values, with R2 at 1.000, and the dynamic range was 0.3–35.0 ng/mL and 0.2–22.0 ng/mL, respectively; the intra- and inter-assay accuracy bias% ranged from −12.2% to −5.2%, precision ranged from −12.4% to −1.4%, and the relative standard deviation (RSD) was less than 6.6% and 8.7%, respectively. The total error was less than 20.4%. The development and validation steps of these two assays met the acceptance criteria for all addressed validation parameters, which suggested that these can be applied to quantify MMAE-conjugated ADCs, as well as in PK studies. Furthermore, these assays can be easily adopted for development of other similar immunoassays.
中文翻译:

用于定量食蟹猴血清中 MMAE 偶联 ADC 和总抗体的酶联免疫吸附试验
抗体-药物偶联物 (ADC) 通常具有异质性,需要在临床前和临床研究中广泛评估暴露-功效和暴露-安全性关系。在本研究中,我们报告了针对单甲基 auristatin E (MMAE) 的单克隆抗体的产生以及灵敏和高通量酶联免疫吸附测定 (ELISA) 的开发、验证和应用,以测量 MMAE 偶联 ADC 的浓度和食蟹猴血清中的总抗体(tAb,ADC 中的抗体加上未结合的抗体)。这些测定成功地应用于 SMADC001 的体外血浆稳定性和药代动力学 (PK) 研究,SMADC001 是一种针对滋养层细胞表面抗原 2 (TROP-2) 的 MMAE 偶联 ADC。SMADC001的血浆稳定性优于与PEG4-Val-Cit偶联的类似ADC,Lys (m-dPEG24)-Cit 和 Val-Cit 接头。开发的 ADC 和 tAb 校准标准的 ELISA 方法揭示了血清浓度和 OD 之间的相关性450 个值,R 2为 1.000,动态范围分别为 0.3–35.0 ng/mL 和 0.2–22.0 ng/mL;批内和批间准确度偏差%范围为-12.2%至-5.2%,精度范围为-12.4%至-1.4%,相对标准偏差(RSD)分别小于6.6%和8.7%。总误差小于20.4%。这两种检测方法的开发和验证步骤符合所有已解决的验证参数的接受标准,这表明这些参数可用于量化 MMAE 偶联 ADC 以及 PK 研究。此外,这些测定法可以很容易地用于开发其他类似的免疫测定法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号