当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Promoting Selective Generation of Formic Acid from CO2 Using Mn(bpy)(CO)3Br as Electrocatalyst and Triethylamine/Isopropanol as Additives
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-23 , DOI: 10.1021/jacs.1c10805 Monica R Madsen 1 , Magnus H Rønne 1 , Marvin Heuschen 1 , Dusanka Golo 2 , Mårten S G Ahlquist 2 , Troels Skrydstrup 1 , Steen U Pedersen 3 , Kim Daasbjerg 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-23 , DOI: 10.1021/jacs.1c10805 Monica R Madsen 1 , Magnus H Rønne 1 , Marvin Heuschen 1 , Dusanka Golo 2 , Mårten S G Ahlquist 2 , Troels Skrydstrup 1 , Steen U Pedersen 3 , Kim Daasbjerg 3
Affiliation
|
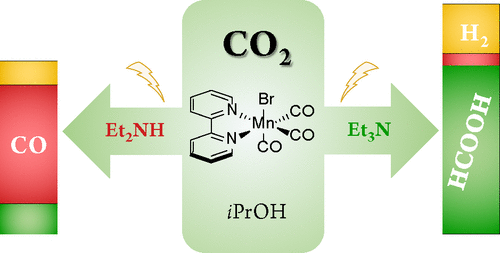
Urgent solutions are needed to efficiently convert the greenhouse gas CO2 into higher-value products. In this work, fac-Mn(bpy)(CO)3Br (bpy = 2,2′-bipyridine) is employed as electrocatalyst in reductive CO2 conversion. It is shown that product selectivity can be shifted from CO toward HCOOH using appropriate additives, i.e., Et3N along with iPrOH. A crucial aspect of the strategy is to outrun the dimer-generating parent-child reaction involving fac-Mn(bpy)(CO)3Br and [Mn(bpy)(CO)3]− and instead produce the Mn hydride intermediate. Preferentially, this is done at the first reduction wave to enable formation of HCOOH at an overpotential as low as 260 mV and with faradaic efficiency of 59 ± 1%. The latter may be increased to 71 ± 3% at an overpotential of 560 mV, using 2 M concentrations of both Et3N and iPrOH. The nature of the amine additive is crucial for product selectivity, as the faradaic efficiency for HCOOH formation decreases to 13 ± 4% if Et3N is replaced with Et2NH. The origin of this difference lies in the ability of Et3N/iPrOH to establish an equilibrium solution of isopropyl carbonate and CO2, while with Et2NH/iPrOH, formation of the diethylcarbamic acid is favored. According to density-functional theory calculations, CO2 in the former case can take part favorably in the catalytic cycle, while this is less opportune in the latter case because of the CO2-to-carbamic acid conversion. This work presents a straightforward procedure for electrochemical reduction of CO2 to HCOOH by combining an easily synthesized manganese catalyst with commercially available additives.
中文翻译:

使用 Mn(bpy)(CO)3Br 作为电催化剂和三乙胺/异丙醇作为添加剂促进从 CO2 中选择性生成甲酸
需要紧急解决方案来有效地将温室气体 CO 2转化为更高价值的产品。在这项工作中,fac -Mn(bpy)(CO) 3 Br(bpy = 2,2'-联吡啶)用作还原 CO 2转化的电催化剂。结果表明,使用适当的添加剂,即 Et 3 N 和i PrOH ,可以将产物选择性从 CO 转变为 HCOOH 。该策略的一个关键方面是超越涉及fac -Mn(bpy)(CO) 3 Br 和 [Mn(bpy)(CO) 3 ] -的产生二聚体的亲子反应而是生产锰氢化物中间体。优选地,这是在第一次还原波中完成的,以便能够在低至 260 mV 的过电位和 59 ± 1% 的法拉第效率下形成 HCOOH。后者可以在 560 mV 的过电位下增加到 71 ± 3%,使用 2 M 浓度的 Et 3 N 和i PrOH。胺添加剂的性质对产品选择性至关重要,因为如果 Et 3 N 被 Et 2 NH取代,HCOOH 形成的法拉第效率会降低至 13 ± 4% 。这种差异的根源在于 Et 3 N/ i PrOH 建立碳酸异丙酯和 CO 2的平衡溶液的能力,而与 Et 2NH /我PrOH中,在形成二乙基氨基甲酸的是有利的。根据密度泛函理论计算,在前一种情况下,CO 2可以有利地参与催化循环,而在后一种情况下,由于 CO 2到氨基甲酸的转化,这种情况不太合适。这项工作通过将易于合成的锰催化剂与市售添加剂相结合,提出了将 CO 2电化学还原为 HCOOH 的简单方法。
更新日期:2021-12-08
中文翻译:

使用 Mn(bpy)(CO)3Br 作为电催化剂和三乙胺/异丙醇作为添加剂促进从 CO2 中选择性生成甲酸
需要紧急解决方案来有效地将温室气体 CO 2转化为更高价值的产品。在这项工作中,fac -Mn(bpy)(CO) 3 Br(bpy = 2,2'-联吡啶)用作还原 CO 2转化的电催化剂。结果表明,使用适当的添加剂,即 Et 3 N 和i PrOH ,可以将产物选择性从 CO 转变为 HCOOH 。该策略的一个关键方面是超越涉及fac -Mn(bpy)(CO) 3 Br 和 [Mn(bpy)(CO) 3 ] -的产生二聚体的亲子反应而是生产锰氢化物中间体。优选地,这是在第一次还原波中完成的,以便能够在低至 260 mV 的过电位和 59 ± 1% 的法拉第效率下形成 HCOOH。后者可以在 560 mV 的过电位下增加到 71 ± 3%,使用 2 M 浓度的 Et 3 N 和i PrOH。胺添加剂的性质对产品选择性至关重要,因为如果 Et 3 N 被 Et 2 NH取代,HCOOH 形成的法拉第效率会降低至 13 ± 4% 。这种差异的根源在于 Et 3 N/ i PrOH 建立碳酸异丙酯和 CO 2的平衡溶液的能力,而与 Et 2NH /我PrOH中,在形成二乙基氨基甲酸的是有利的。根据密度泛函理论计算,在前一种情况下,CO 2可以有利地参与催化循环,而在后一种情况下,由于 CO 2到氨基甲酸的转化,这种情况不太合适。这项工作通过将易于合成的锰催化剂与市售添加剂相结合,提出了将 CO 2电化学还原为 HCOOH 的简单方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号