Journal of Catalysis ( IF 7.3 ) Pub Date : 2021-11-23 , DOI: 10.1016/j.jcat.2021.11.024 Uta Hejral 1 , Janis Timoshenko 1 , David Kordus 1, 2 , Mauricio Lopez Luna 1 , Nuria J. Divins 2 , Simon Widrinna 1, 2 , Ioannis Zegkinoglou 2 , Lukas Pielsticker 2 , Hemma Mistry 2 , Jorge Anibal Boscoboinik 3 , Stefanie Kuehl 1 , Beatriz Roldan Cuenya 1
|
The hydrogenation of CO2 into high energy density fuels such as methanol, where the required H2 is obtained from renewable sources, is of utmost importance for a sustainable society. In recent years, NiGa alloys have attracted attention as promising catalyst material systems for the hydrogenation of CO2 into methanol at ambient pressures. They thus represent an energy-saving alternative to the Cu-based catalysts employed in today’s catalytic industry that require high pressures for the CO2 hydrogenation. However, the underlying reaction mechanisms for the NiGa system are still under debate. One of the challenges here is to unravel the evolution and coexistence of the different species in the heterogeneous NiGa catalyst system under activation and reaction conditions. To shed light on their evolution under activation in H2 and their catalytic roles under CO2 hydrogenation working conditions on well-defined Ni3Ga1 and Ni5Ga3 nanoparticle (NP) catalysts, we employed a multi-probe approach. It included advanced machine learning-based analysis of operando X-ray absorption spectroscopy data combined with operando powder X-ray diffraction and near ambient pressure X-ray photoelectron spectroscopy measurements, as well as reactivity studies using bed-packed mass flow reactors. In addition, we employed atomic force microscopy and scanning transmission electron microscopy for structural characterization.
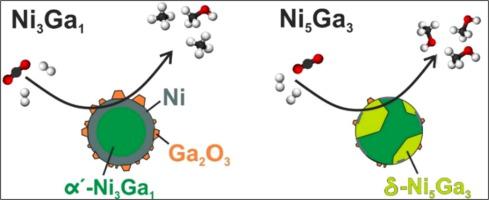
Under H2 activation at 1 bar total pressure, we concluded the formation of metallic Ni, starting for Ni3Ga1 at 300 °C, and for Ni5Ga3 at 400 °C. At higher temperatures, the formation of NiGa alloys follows. The α’-Ni3Ga1 alloy phase is predominantly formed for the Ni3Ga1 NPs, while the coexistence of α’-Ni3Ga1, δ-Ni5Ga3 and Ga2O3 phases is observed for the Ni5Ga3 NPs after the H2 activation. The formation of the Ga2O3 phase also results in the presence of excess metallic Ni. Under CO2 hydrogenation reaction conditions, Ga partially oxidizes again to form a Ga2O3-rich particle shell for both NP compositions, yet, to a larger extent for the Ni3Ga1 NPs, which, in turn, feature a higher amount of excess Ni. We reveal that metallic Ni is responsible for the high selectivity of the Ni3Ga1 NPs towards the production of methane in our catalytic tests. In contrast, the Ni5Ga3 NPs display a strong selectivity toward methanol production (>92%), more than one order of magnitude higher than that for the Ni3Ga1 NPs, which we ascribe to the presence of the δ-Ni5Ga3 phase.
中文翻译:

在活化和工作条件下跟踪用于甲醇合成的胶束基 NiGa 纳米催化剂的相变
将 CO 2氢化成高能量密度燃料(例如甲醇),其中所需的 H 2来自可再生资源,这对于可持续社会至关重要。近年来,NiGa合金作为用于在环境压力下将CO 2加氢成甲醇的有前途的催化剂材料系统而受到关注。因此,它们代表了当今催化行业使用的铜基催化剂的节能替代品,这些催化剂需要高压处理 CO 2氢化。然而,NiGa 系统的潜在反应机制仍在争论中。这里的挑战之一是在活化和反应条件下解开多相 NiGa 催化剂体系中不同物种的演变和共存。为了阐明它们在 H 2 中活化下的演化以及它们在 CO 2加氢工作条件下对明确定义的 Ni 3 Ga 1和 Ni 5 Ga 3纳米颗粒 (NP) 催化剂的催化作用,我们采用了多探针方法。它包括基于操作数X 射线吸收光谱数据的高级机器学习分析,并结合操作数粉末 X 射线衍射和近环境压力 X 射线光电子能谱测量,以及使用填充床质量流反应器的反应性研究。此外,我们采用原子力显微镜和扫描透射电子显微镜进行结构表征。
在1 bar 总压力下的 H 2活化下,我们得出金属 Ni 的形成,从300 °C 的Ni 3 Ga 1开始,以及在 400 °C 的Ni 5 Ga 3开始。在更高的温度下,NiGa合金的形成随之而来。Ni 3 Ga 1 NPs主要形成α'-Ni 3 Ga 1合金相,而Ni观察到α'-Ni 3 Ga 1、δ-Ni 5 Ga 3和Ga 2 O 3相共存H 2后的5 Ga 3 NP激活。Ga 2 O 3相的形成也导致过量金属Ni的存在。在 CO 2加氢反应条件下,对于两种 NP 组合物,Ga 再次部分氧化以形成富含Ga 2 O 3 的粒子壳,但对于 Ni 3 Ga 1 NPs 的程度更大,反过来,Ni 3 Ga 1 NPs 具有更高的含量过量的 Ni。我们发现,在我们的催化测试中,金属 Ni 是 Ni 3 Ga 1 NPs 对甲烷产生的高选择性的原因。相比之下,Ni 5 Ga 3NPs 对甲醇生产表现出很强的选择性 (>92%),比 Ni 3 Ga 1 NPs高一个数量级以上,我们将其归因于 δ-Ni 5 Ga 3相的存在。


























 京公网安备 11010802027423号
京公网安备 11010802027423号