当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The electron attachment effect on the structure and properties of ortho-hydroxyaryl Schiff and Mannich bases – the hydrogen/proton transfer processes
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-04 , DOI: 10.1039/d1cp03723d Jerzy J Jański 1 , Szczepan Roszak 2 , Kazimierz Orzechowski 1 , Lucjan Sobczyk 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-11-04 , DOI: 10.1039/d1cp03723d Jerzy J Jański 1 , Szczepan Roszak 2 , Kazimierz Orzechowski 1 , Lucjan Sobczyk 1
Affiliation
|
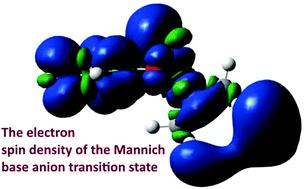
The attachment of electrons is known to significantly influence some chemical and biological processes. The chemical differences between Schiff and Mannich bases are characterized by strong intramolecular hydrogen bonds, resulting from the presence of, respectively, single or double carbon–nitrogen bonds in the chelate rings. Differences are especially visible in the hydrogen transfer processes from molecular (O–H⋯N) to the proton transfer (O⋯H–N) forms. The reaction in a Schiff base occurs as an ordinary hydrogen transfer from a donor to an acceptor, while in a Mannich base the transfer of hydrogen occurs simultaneously with a C–N bond scission leading to an intermolecular complex. The attachment of electrons preserves the overall structural topology of the reactants; however, due to differences in electron affinities, reactions switch from endothermic to exothermic and reaction rates in the anionic systems are significantly higher. The difference in electron affinities for particular reactants comes from the fundamental differences in electron binding mechanisms, leading to the valence-bound or dipole-bound states. The observed mechanisms are closely related to the nature and size of the LUMOs of the parent molecules. The transition state of the Mannich base corresponds to the σ and π orbital conversion and possesses the characteristics of the valence-bound state and the dipole-bound electronic state.
中文翻译:

电子附着对邻羟基芳基席夫碱和曼尼希碱的结构和性质的影响——氢/质子转移过程
众所周知,电子的附着会显着影响一些化学和生物过程。席夫碱和曼尼希碱之间的化学差异以分子内强氢键为特征,这是由于螯合环中分别存在单碳-氮键或双碳-氮键所致。在从分子(O-H⋯N)到质子转移(O⋯H-N)形式的氢转移过程中,差异尤其明显。席夫碱中的反应发生为从供体到受体的普通氢转移,而在曼尼希碱中,氢的转移与导致分子间复合物的 C-N 键断裂同时发生。电子的附着保留了反应物的整体结构拓扑;然而,由于电子亲和力的不同,反应从吸热转变为放热,阴离子体系中的反应速率显着提高。特定反应物的电子亲和力差异来自于电子结合机制的根本差异,导致了价结合或偶极结合状态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。导致价束缚态或偶极束缚态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。导致价束缚态或偶极束缚态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。
更新日期:2021-11-23
中文翻译:

电子附着对邻羟基芳基席夫碱和曼尼希碱的结构和性质的影响——氢/质子转移过程
众所周知,电子的附着会显着影响一些化学和生物过程。席夫碱和曼尼希碱之间的化学差异以分子内强氢键为特征,这是由于螯合环中分别存在单碳-氮键或双碳-氮键所致。在从分子(O-H⋯N)到质子转移(O⋯H-N)形式的氢转移过程中,差异尤其明显。席夫碱中的反应发生为从供体到受体的普通氢转移,而在曼尼希碱中,氢的转移与导致分子间复合物的 C-N 键断裂同时发生。电子的附着保留了反应物的整体结构拓扑;然而,由于电子亲和力的不同,反应从吸热转变为放热,阴离子体系中的反应速率显着提高。特定反应物的电子亲和力差异来自于电子结合机制的根本差异,导致了价结合或偶极结合状态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。导致价束缚态或偶极束缚态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。导致价束缚态或偶极束缚态。观察到的机制与母体分子的 LUMO 的性质和大小密切相关。曼尼希碱基的过渡态对应于σ和π轨道转换,具有价束缚态和偶极束缚电子态的特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号