当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the mechanism of the arylation of arenes via norbornene relay palladation through meta- to para-selectivity
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1qo01500a Shengnan Liu 1 , Qiong Wang 1 , Fang Huang 1 , Wenjuan Wang 1 , Chong Yang 1 , Jianbiao Liu 1 , Dezhan Chen 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1qo01500a Shengnan Liu 1 , Qiong Wang 1 , Fang Huang 1 , Wenjuan Wang 1 , Chong Yang 1 , Jianbiao Liu 1 , Dezhan Chen 1
Affiliation
|
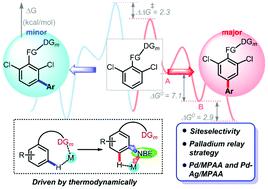
A novel mechanism of the arylation of arenes via norbornene (NBE) relay palladation through meta- to para-selectivity was revealed via density functional theory (DFT) calculations. Our calculated results revealed that the reaction was initiated by a [mono-N-protected amino acid ligand (MPAA)–Pd] complex to activate at first the meta-C–H guided by the directing group (DG), and para-arylation was subsequently achieved by NBE relay palladation from meta- to para-position. Significantly, the palladium/norbornene (Pd/NBE) cooperative catalysis was catalyzed by a Pd–Ag bimetallic complex, which accounted for the experimental fact that no yield detected without Ag. The reaction pathway through para- to meta-selectivity was also investigated, while this pathway was kinetically unfavorable. The results revealed that the initial DG guided C–H site activation was the rate-determining step and played an important role in determining site-selectivity. The primary meta-activation was favorable in energy due to the less ring strain in the cyclic nitrile-coordinated C–H transition states in the meta position. Moreover, the perfect cooperation of a remote directing template and a transient mediator NBE through the alternating association with the Pd center achieved the relay through meta- to para-position. The present results provide a reasonable insight into the para-C–H arylation by the Pd/MPAA/NBE cooperative catalysis in conjunction with a precise DG and Ag(I) additive.
中文翻译:

通过间位到对位选择性通过降冰片烯中继钯化对芳烃芳基化机制的深入了解
芳烃的芳基化的一种新的机制通过降冰片烯(NBE)中继palladation通过间位-到对位-选择性揭示通过密度泛函理论(DFT)计算。我们的计算结果表明,反应是由 [单-N-保护的氨基酸配体 (MPAA)-Pd] 复合物引发的,首先激活由导向基团 (DG) 引导的间位-C-H,然后对位芳基化随后通过 NBE 从元到对的中继 palladation 实现-位置。值得注意的是,钯/降冰片烯 (Pd/NBE) 协同催化是由 Pd-Ag 双金属配合物催化的,这说明了在没有 Ag 的情况下没有检测到产率的实验事实。还研究了通过对位选择性到间位选择性的反应途径,而该途径在动力学上是不利的。结果表明,初始 DG 引导的 C-H 位点激活是速率决定步骤,并在确定位点选择性方面发挥重要作用。主元-activation是能量有利的由于在循环腈配位C-H过渡态少环张力元位置。此外,通过与Pd中心的交替关联,远程定向模板和瞬态中介NBE的完美配合实现了元到对位的中继。目前的结果为Pd/MPAA/NBE 协同催化结合精确的 DG 和 Ag( I ) 添加剂对对-C-H 芳基化提供了合理的见解。
更新日期:2021-12-01
中文翻译:

通过间位到对位选择性通过降冰片烯中继钯化对芳烃芳基化机制的深入了解
芳烃的芳基化的一种新的机制通过降冰片烯(NBE)中继palladation通过间位-到对位-选择性揭示通过密度泛函理论(DFT)计算。我们的计算结果表明,反应是由 [单-N-保护的氨基酸配体 (MPAA)-Pd] 复合物引发的,首先激活由导向基团 (DG) 引导的间位-C-H,然后对位芳基化随后通过 NBE 从元到对的中继 palladation 实现-位置。值得注意的是,钯/降冰片烯 (Pd/NBE) 协同催化是由 Pd-Ag 双金属配合物催化的,这说明了在没有 Ag 的情况下没有检测到产率的实验事实。还研究了通过对位选择性到间位选择性的反应途径,而该途径在动力学上是不利的。结果表明,初始 DG 引导的 C-H 位点激活是速率决定步骤,并在确定位点选择性方面发挥重要作用。主元-activation是能量有利的由于在循环腈配位C-H过渡态少环张力元位置。此外,通过与Pd中心的交替关联,远程定向模板和瞬态中介NBE的完美配合实现了元到对位的中继。目前的结果为Pd/MPAA/NBE 协同催化结合精确的 DG 和 Ag( I ) 添加剂对对-C-H 芳基化提供了合理的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号