当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dibenzocycloheptanones construction through a removable P-centered radical: synthesis of allocolchicine analogues
Chemical Science ( IF 8.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1sc05404j Yan Zhang 1 , Zhenzhi Cai 1 , Julia Struwe 2 , Chanchan Ma 1 , Wangyu Zeng 1 , Xinyi Liao 1 , Min Xu 1 , Lutz Ackermann 2
Chemical Science ( IF 8.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1sc05404j Yan Zhang 1 , Zhenzhi Cai 1 , Julia Struwe 2 , Chanchan Ma 1 , Wangyu Zeng 1 , Xinyi Liao 1 , Min Xu 1 , Lutz Ackermann 2
Affiliation
|
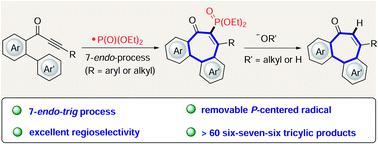
Dibenzocycloheptanones containing a tricyclic 6–7–6-system are present in numerous biologically active natural molecules. However, the simple and efficient preparation of derivatives containing a dibenzocycloheptanone scaffold remains difficult to date. Herein, we report a versatile strategy for the construction of these challenging seven-membered rings using a 7-endo-trig cyclization which is initiated by a phosphorus-centered radical. This approach provides a step-economical regime for the facile assembly of a wide range of phosphorylated dibenzocycloheptanones. Remarkably, we also have devised a traceless addition/exchange strategy for the preparation of dephosphorylated products at room temperature with excellent yields. Therefore, this protocol allows for the concise synthesis of biorelevant allocochicine derivatives.
中文翻译:

通过可去除的 P 中心自由基构建二苯并环庚酮:别秋水仙碱类似物的合成
含有三环 6-7-6-系统的二苯并环庚酮存在于许多具有生物活性的天然分子中。然而,迄今为止,简单有效地制备含有二苯并环庚酮支架的衍生物仍然很困难。在这里,我们报告了一种使用 7- endo构建这些具有挑战性的七元环的通用策略-trig 环化,由以磷为中心的自由基引发。这种方法为轻松组装各种磷酸化二苯并环庚酮提供了一种经济的方法。值得注意的是,我们还设计了一种无痕添加/交换策略,用于在室温下以优异的收率制备去磷酸化产物。因此,该协议允许简明地合成生物相关的 allocochicine 衍生物。
更新日期:2021-11-22
中文翻译:

通过可去除的 P 中心自由基构建二苯并环庚酮:别秋水仙碱类似物的合成
含有三环 6-7-6-系统的二苯并环庚酮存在于许多具有生物活性的天然分子中。然而,迄今为止,简单有效地制备含有二苯并环庚酮支架的衍生物仍然很困难。在这里,我们报告了一种使用 7- endo构建这些具有挑战性的七元环的通用策略-trig 环化,由以磷为中心的自由基引发。这种方法为轻松组装各种磷酸化二苯并环庚酮提供了一种经济的方法。值得注意的是,我们还设计了一种无痕添加/交换策略,用于在室温下以优异的收率制备去磷酸化产物。因此,该协议允许简明地合成生物相关的 allocochicine 衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号