当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Potential of 2-(2-Nitrophenyl)ethyl-Caged N-Hydroxysulfonamides for the Photoactivated Release of Nitroxyl (HNO)
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-19 , DOI: 10.1021/acs.joc.1c01800 Vinay Bharadwaj 1, 2 , Mohammad S Rahman 3 , Paul Sampson 3 , Alexander J Seed 3 , Nicola E Brasch 1, 2, 4
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-19 , DOI: 10.1021/acs.joc.1c01800 Vinay Bharadwaj 1, 2 , Mohammad S Rahman 3 , Paul Sampson 3 , Alexander J Seed 3 , Nicola E Brasch 1, 2, 4
Affiliation
|
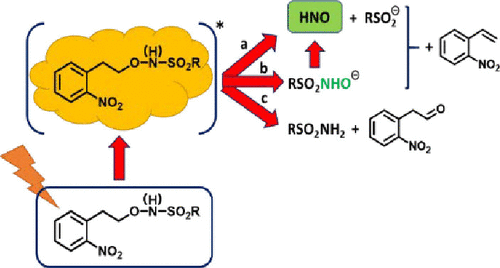
The emergence of nitroxyl (HNO) as a biological signaling molecule is attracting increasing attention. HNO-based prodrugs show considerable potential in treating congestive heart failure, with HNO reacting rapidly with metal centers and protein-bound and free thiols. A new class of 2-(2-nitrophenyl)ethyl (2-NPE)-photocaged N-hydroxysulfonamides has been developed, and the mechanisms of photodecomposition have been investigated. Three photodecomposition pathways are observed: the desired concomitant C–O/N–S bond cleavage to generate HNO, sulfinate, and 2-nitrostyrene, C–O bond cleavage to give the parent sulfohydroxamic acid and 2-nitrostyrene, and O–N bond cleavage to release a sulfonamide and 2-nitrophenylacetaldehyde. Laser flash photolysis experiments provide support for a Norrish type II mechanism involving 1,5-hydrogen atom abstraction to generate an aci-nitro species. A mechanism is proposed in which the (Z)-aci-nitro intermediate undergoes either C–O bond cleavage to release RSO2NHO(H), concerted C–O/N–S bond cleavage to generate sulfinate and HNO, or isomerization to the (E)-isomer prior to O–N bond cleavage. The pKa of the N(H) of the N-hydroxysulfonamide plays a key role in determining whether C–O or concerted C–O/N–S bond cleavage occurs. Deprotonating this site favors the desired C–O/N–S bond cleavage at the expense of an increased level of undesired O–N bond cleavage. Triplet state quenchers have no effect on the observed photoproducts.
中文翻译:

探索 2-(2-Nitrophenyl)ethyl-Caged N-Hydroxysulfonamides 光活化释放硝酰 (HNO) 的潜力
硝酰基(HNO)作为一种生物信号分子的出现引起了越来越多的关注。基于 HNO 的前药在治疗充血性心力衰竭方面显示出相当大的潜力,HNO 与金属中心和蛋白质结合和游离硫醇迅速反应。一类新的2-(2-硝基苯基)乙基(2-NPE)-光笼N已开发出β-羟基磺胺类药物,并研究了光分解的机理。观察到三种光分解途径:所需的伴随 C-O/N-S 键断裂产生 HNO、亚磺酸盐和 2-硝基苯乙烯,C-O 键断裂产生母体磺基异羟肟酸和 2-硝基苯乙烯,以及 O-N 键裂解释放出磺胺和2-硝基苯乙醛。激光闪光光解实验为涉及 1,5-氢原子提取以生成aci - nitro 物种的 Norrish II 型机制提供了支持。提出了一种机制,其中 ( Z ) -aci - nitro 中间体经历 C-O 键裂解以释放 RSO 2NHO(H),协同 C-O/N-S 键断裂生成亚磺酸盐和 HNO,或在 O-N 键断裂之前异构化为 ( E )-异构体。N-羟基磺酰胺的 N(H) 的p K a在确定是否发生 C-O 或协同 C-O/N-S 键断裂中起关键作用。去质子化该位点有利于所需的 C-O/N-S 键断裂,但会增加不希望的 O-N 键断裂水平。三重态猝灭剂对观察到的光产物没有影响。
更新日期:2021-12-03
中文翻译:

探索 2-(2-Nitrophenyl)ethyl-Caged N-Hydroxysulfonamides 光活化释放硝酰 (HNO) 的潜力
硝酰基(HNO)作为一种生物信号分子的出现引起了越来越多的关注。基于 HNO 的前药在治疗充血性心力衰竭方面显示出相当大的潜力,HNO 与金属中心和蛋白质结合和游离硫醇迅速反应。一类新的2-(2-硝基苯基)乙基(2-NPE)-光笼N已开发出β-羟基磺胺类药物,并研究了光分解的机理。观察到三种光分解途径:所需的伴随 C-O/N-S 键断裂产生 HNO、亚磺酸盐和 2-硝基苯乙烯,C-O 键断裂产生母体磺基异羟肟酸和 2-硝基苯乙烯,以及 O-N 键裂解释放出磺胺和2-硝基苯乙醛。激光闪光光解实验为涉及 1,5-氢原子提取以生成aci - nitro 物种的 Norrish II 型机制提供了支持。提出了一种机制,其中 ( Z ) -aci - nitro 中间体经历 C-O 键裂解以释放 RSO 2NHO(H),协同 C-O/N-S 键断裂生成亚磺酸盐和 HNO,或在 O-N 键断裂之前异构化为 ( E )-异构体。N-羟基磺酰胺的 N(H) 的p K a在确定是否发生 C-O 或协同 C-O/N-S 键断裂中起关键作用。去质子化该位点有利于所需的 C-O/N-S 键断裂,但会增加不希望的 O-N 键断裂水平。三重态猝灭剂对观察到的光产物没有影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号