当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Expanding the Conformational Landscape of Minimalistic Tripeptides by Their O-Glycosylation
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-19 , DOI: 10.1021/jacs.1c07592 Alexandra Brito 1, 2, 3 , Dhwanit Dave 3, 4, 5 , Ayala Lampel 3 , Vânia I B Castro 1, 2 , Daniela Kroiss 3, 4, 5 , Rui L Reis 1, 2 , Tell Tuttle 6 , Rein V Ulijn 3, 4, 5, 7 , Ricardo A Pires 1, 2 , Iva Pashkuleva 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-11-19 , DOI: 10.1021/jacs.1c07592 Alexandra Brito 1, 2, 3 , Dhwanit Dave 3, 4, 5 , Ayala Lampel 3 , Vânia I B Castro 1, 2 , Daniela Kroiss 3, 4, 5 , Rui L Reis 1, 2 , Tell Tuttle 6 , Rein V Ulijn 3, 4, 5, 7 , Ricardo A Pires 1, 2 , Iva Pashkuleva 1, 2
Affiliation
|
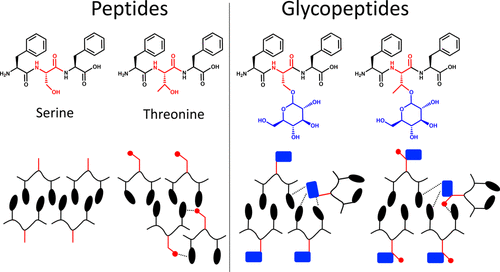
We report on the supramolecular self-assembly of tripeptides and their O-glycosylated analogues, in which the carbohydrate moiety is coupled to a central serine or threonine flanked by phenylalanine residues. The substitution of serine with threonine introduces differential side-chain interactions, which results in the formation of aggregates with different morphology. O-glycosylation decreases the aggregation propensity because of rebalancing of the π interactions. The glycopeptides form aggregates with reduced stiffness but increased thermal stability. Our results demonstrate that the designed minimalistic glycopeptides retain critical functional features of glycoproteins and therefore are promising tools for elucidation of molecular mechanisms involved in the glycoprotein interactome. They can also serve as an inspiration for the design of functional glycopeptide-based biomaterials.
中文翻译:

通过 O-糖基化扩展简约三肽的构象景观
我们报告了三肽及其O-糖基化类似物的超分子自组装,其中碳水化合物部分与苯丙氨酸残基两侧的中心丝氨酸或苏氨酸偶联。用苏氨酸取代丝氨酸引入了不同的侧链相互作用,导致形成具有不同形态的聚集体。○由于 π 相互作用的重新平衡,-糖基化降低了聚集倾向。糖肽形成具有降低的刚度但增加的热稳定性的聚集体。我们的结果表明,设计的简约糖肽保留了糖蛋白的关键功能特征,因此是阐明糖蛋白相互作用组中涉及的分子机制的有前途的工具。它们还可以作为设计基于功能性糖肽的生物材料的灵感。
更新日期:2021-12-01
中文翻译:

通过 O-糖基化扩展简约三肽的构象景观
我们报告了三肽及其O-糖基化类似物的超分子自组装,其中碳水化合物部分与苯丙氨酸残基两侧的中心丝氨酸或苏氨酸偶联。用苏氨酸取代丝氨酸引入了不同的侧链相互作用,导致形成具有不同形态的聚集体。○由于 π 相互作用的重新平衡,-糖基化降低了聚集倾向。糖肽形成具有降低的刚度但增加的热稳定性的聚集体。我们的结果表明,设计的简约糖肽保留了糖蛋白的关键功能特征,因此是阐明糖蛋白相互作用组中涉及的分子机制的有前途的工具。它们还可以作为设计基于功能性糖肽的生物材料的灵感。


























 京公网安备 11010802027423号
京公网安备 11010802027423号