当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative Asymmetric Cation-Binding Catalysis
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.accounts.1c00400 Amol P Jadhav 1 , Sang Yeon Park 1 , Ji-Woong Lee 2 , Hailong Yan 3 , Choong Eui Song 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.accounts.1c00400 Amol P Jadhav 1 , Sang Yeon Park 1 , Ji-Woong Lee 2 , Hailong Yan 3 , Choong Eui Song 1
Affiliation
|
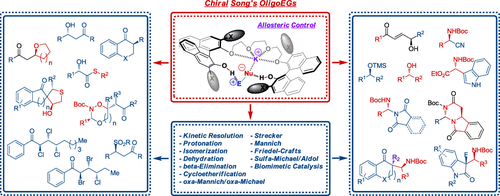
Asymmetric cation-binding catalysis in principle enables the use of (alkali) metal salts, otherwise insoluble in organic solvents, as reagents and effectors in enantioselective reactions. However, this concept has been a formidable challenge due to the difficulties associated with creating a highly organized chiral environment for cations and anions simultaneously. Over the last four decades, various chiral crown ethers have been developed as cation-binding phase-transfer catalysts and examined in asymmetric catalysis. However, the limited ability of chiral crown ethers to generate soluble reactive anions in a confined chiral cage offers a restricted reaction scope and unsatisfactory chirality induction. To address the constraints of monofunctional chiral crown ethers as cation-binding catalysts, it is therefore desirable to develop a cooperative cation-binding catalyst possessing secondary binding sites for anions, which enables the generation of a reactive anion within a chiral cage of a catalyst. This account summarizes our design, development, and applications of chiral BINOL-based oligoethylene glycols (oligoEGs) as a new type of bifunctional cation-binding catalyst. We initially found that achiral oligoEGs were efficient promoters in nucleophilic fluorination with potassium fluoride. Thereby, we hypothesized that, by breaking the closed cyclic ether unit of chiral crown ethers, the free terminal −OH groups could activate the electrophiles by hydrogen bonding whereas the ether oxygens could act as the Lewis base to coordinate metal ions, thus generating soluble anions in a confined chiral cage. This hypothesis was realized by synthesizing a series of chiral variants of oligoEGs by connecting two 3,3′-disubstituted-BINOL units with glycol linkers. Readily available BINOL-based chiral oligoEGs enabled numerous asymmetric transformations out of the reach of chiral monofunctional crown ether catalysts. We have demonstrated that this new type of bifunctional cation-binding catalysts can generate a soluble fluoride anion from alkali metal fluorides, which can be a versatile chiral promoter for diverse asymmetric catalytic reactions, kinetic resolution (selectivity factor of up to ∼2300), asymmetric protonation, Mannich reactions, tandem cyclization reactions, and the isomerization of allylic alcohols and hemithioacetals. We have also successfully utilized our chiral oligoEG catalysts along with alkali metal salts of carbon- and heteroatom-based nucleophiles, respectively, for asymmetric Strecker reactions and the asymmetric synthesis of chiral aminals. The power of our cooperative cation-binding catalysis was exemplified by kinetic resolution reactions of secondary alcohols, achieving highly enantioselective catalysis with only <1 ppm loading of an organocatalyst with high TOFs (up to ∼1300 h–1 at 1 ppm catalyst loading). The broadness and generality of our cooperative asymmetric cation-binding catalysis can be ascribed, in a similar fashion, to active-site architectures of enzymes using allosteric interactions, highly confined chiral cages formed by the incorporation of alkali metal salts in the catalyst polyether chain backbone, and the cooperative activation of reacting partners by hydrogen-bonding and ion–ion interactions. Confining reactive components in such a chiral binding pocket leads to enhanced reactivity and efficient transfer of the stereochemical information.
中文翻译:

协同不对称阳离子结合催化
不对称阳离子结合催化原则上能够使用(碱)金属盐,否则不溶于有机溶剂,作为对映选择性反应的试剂和效应物。然而,由于难以同时为阳离子和阴离子创造高度有序的手性环境,这一概念一直是一项艰巨的挑战。在过去的四年中,各种手性冠醚已被开发为阳离子结合相转移催化剂,并在不对称催化中进行了检验。然而,手性冠醚在受限的手性笼中产生可溶性活性阴离子的能力有限,这限制了反应范围和不令人满意的手性诱导。为了解决单官能手性冠醚作为阳离子结合催化剂的限制,因此,需要开发一种具有阴离子二级结合位点的协同阳离子结合催化剂,它能够在催化剂的手性笼内产生反应性阴离子。本文总结了我们将手性 BINOL 基低聚乙二醇 (oligoEGs) 作为新型双功能阳离子结合催化剂的设计、开发和应用。我们最初发现非手性 oligoEGs 是氟化钾亲核氟化的有效促进剂。因此,我们假设,通过破坏手性冠醚的封闭环醚单元,自由末端-OH基团可以通过氢键激活亲电试剂,而醚氧可以作为路易斯碱来配位金属离子,从而产生可溶性阴离子在一个封闭的手性笼子里。该假设是通过将两个 3,3'-二取代-BINOL 单元与乙二醇接头连接来合成一系列 oligoEG 的手性变体来实现的。现成的基于 BINOL 的手性 oligoEG 能够实现手性单官能冠醚催化剂无法实现的许多不对称转化。我们已经证明,这种新型双功能阳离子结合催化剂可以从碱金属氟化物中产生可溶性氟化物阴离子,可以作为多种不对称催化反应的通用手性促进剂、动力学分辨率(高达 ∼2300 的选择性因子)、不对称质子化、曼尼希反应、串联环化反应以及烯丙醇和半硫缩醛的异构化。我们还成功地利用我们的手性 oligoEG 催化剂以及碳基和杂原子基亲核试剂的碱金属盐,分别用于不对称 Strecker 反应和手性缩醛胺的不对称合成。我们的协同阳离子结合催化的力量体现在仲醇的动力学拆分反应中,实现了高度对映选择性催化,只需 <1 ppm 的高 TOF 有机催化剂负载(高达 ∼1300 小时)–1在 1 ppm 催化剂负载下)。我们合作的不对称阳离子结合催化的广泛性和普遍性可以以类似的方式归因于使用变构相互作用的酶的活性位点结构,通过在催化剂聚醚链骨架中掺入碱金属盐形成的高度受限的手性笼,以及通过氢键和离子 - 离子相互作用协同激活反应伙伴。将反应性组分限制在这种手性结合袋中会导致立体化学信息的反应性增强和有效传递。
更新日期:2021-12-07
中文翻译:

协同不对称阳离子结合催化
不对称阳离子结合催化原则上能够使用(碱)金属盐,否则不溶于有机溶剂,作为对映选择性反应的试剂和效应物。然而,由于难以同时为阳离子和阴离子创造高度有序的手性环境,这一概念一直是一项艰巨的挑战。在过去的四年中,各种手性冠醚已被开发为阳离子结合相转移催化剂,并在不对称催化中进行了检验。然而,手性冠醚在受限的手性笼中产生可溶性活性阴离子的能力有限,这限制了反应范围和不令人满意的手性诱导。为了解决单官能手性冠醚作为阳离子结合催化剂的限制,因此,需要开发一种具有阴离子二级结合位点的协同阳离子结合催化剂,它能够在催化剂的手性笼内产生反应性阴离子。本文总结了我们将手性 BINOL 基低聚乙二醇 (oligoEGs) 作为新型双功能阳离子结合催化剂的设计、开发和应用。我们最初发现非手性 oligoEGs 是氟化钾亲核氟化的有效促进剂。因此,我们假设,通过破坏手性冠醚的封闭环醚单元,自由末端-OH基团可以通过氢键激活亲电试剂,而醚氧可以作为路易斯碱来配位金属离子,从而产生可溶性阴离子在一个封闭的手性笼子里。该假设是通过将两个 3,3'-二取代-BINOL 单元与乙二醇接头连接来合成一系列 oligoEG 的手性变体来实现的。现成的基于 BINOL 的手性 oligoEG 能够实现手性单官能冠醚催化剂无法实现的许多不对称转化。我们已经证明,这种新型双功能阳离子结合催化剂可以从碱金属氟化物中产生可溶性氟化物阴离子,可以作为多种不对称催化反应的通用手性促进剂、动力学分辨率(高达 ∼2300 的选择性因子)、不对称质子化、曼尼希反应、串联环化反应以及烯丙醇和半硫缩醛的异构化。我们还成功地利用我们的手性 oligoEG 催化剂以及碳基和杂原子基亲核试剂的碱金属盐,分别用于不对称 Strecker 反应和手性缩醛胺的不对称合成。我们的协同阳离子结合催化的力量体现在仲醇的动力学拆分反应中,实现了高度对映选择性催化,只需 <1 ppm 的高 TOF 有机催化剂负载(高达 ∼1300 小时)–1在 1 ppm 催化剂负载下)。我们合作的不对称阳离子结合催化的广泛性和普遍性可以以类似的方式归因于使用变构相互作用的酶的活性位点结构,通过在催化剂聚醚链骨架中掺入碱金属盐形成的高度受限的手性笼,以及通过氢键和离子 - 离子相互作用协同激活反应伙伴。将反应性组分限制在这种手性结合袋中会导致立体化学信息的反应性增强和有效传递。



























 京公网安备 11010802027423号
京公网安备 11010802027423号